Intake to Insight on a
Unified Safety Platform
Case processing, signal detection, advanced reporting, and
safety content management.
Veeva Safety enables biotechs to work more effectively
Veeva Safety Suite
Safety Suite of applications operate as a unified pharmacovigilance system on a single cloud platform to maximize operational efficiencies and improve patient safety.
Safety applications share a common data model which enables the end-to-end management of safety processes.
Veeva Safety
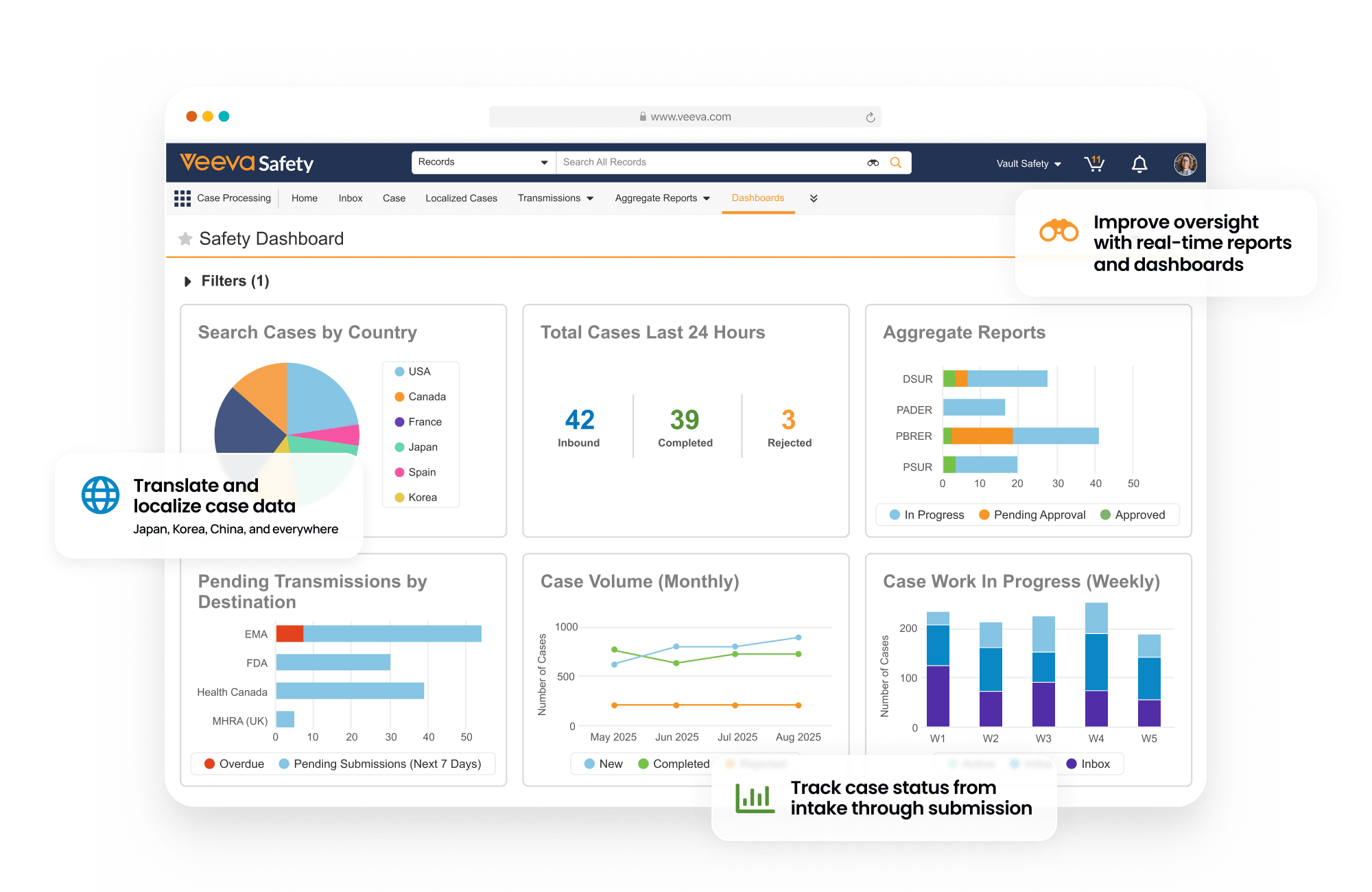
Global safety case intake, processing, and ICSR reporting.
Veeva SafetyDocs
Safety / pharmacovigilance-related content and process management.
Veeva Safety Workbench
Reporting and analytics application that requires Veeva Safety.
Veeva Safety Signal
Signal detection application that requires Veeva Safety.
Veeva AI for Safety
See in Action
Case Intake Agent
Automates case intake for faster, more accurate data extraction and identifies potential data anomalies.
Case Narrative Agent
Enhance case narratives by correcting grammar, consolidating information, and improving readability.
Veeva Connections
Veeva Connections are Veeva-delivered integrations that seamlessly transfer data and documents.
Safety-EDC Connection
Automates flow of clinical serious adverse event (SAE) data, and provides safety teams direct access to subject data.
Safety-Clinical Operations Connection
Enables seamless flow of safety letters to Veeva eTMF, and with CTMS and SiteConnect, distribution to sites with tracking.
Medical-Safety Connection
Streamlines intake of adverse event data from medical.
Safety–RIM Connection
Shares registered product data.
See a full list of available Veeva Connections.