Unification is Top Priority for Clinical Operations
More than 250 global, clinical operations professionals came together at the fourth Veeva R&D Summit this month in Philadelphia. Two key themes dominated discussions; unified clinical and the move from passive to active TMF.
J&J Innovative Medicine’s Terry Murphy kicked-off the clinical sessions by detailing his company’s approach to unifying clinical operations on a single platform. Coming from a manufacturing world before joining J&J Innovative Medicine, Terry was “stunned by the amount of paperwork still moving around.” He talked about the benefits of implementing a wholesale change, including leveraging real-time data for better patient engagement, proactive trial adjustment, and ultimately, delivering cures faster.
In a separate session, Ed Leftin discussed Ora, Inc’s journey to modernize and unify their clinical technology landscape. He described some of the challenges that drove them to find a better way to manage clinical trials – such as spreadsheets to track spreadsheets. The audience nodded in agreement at a slide depicting how clinical operating environments typically look prior to unifying clinical systems.
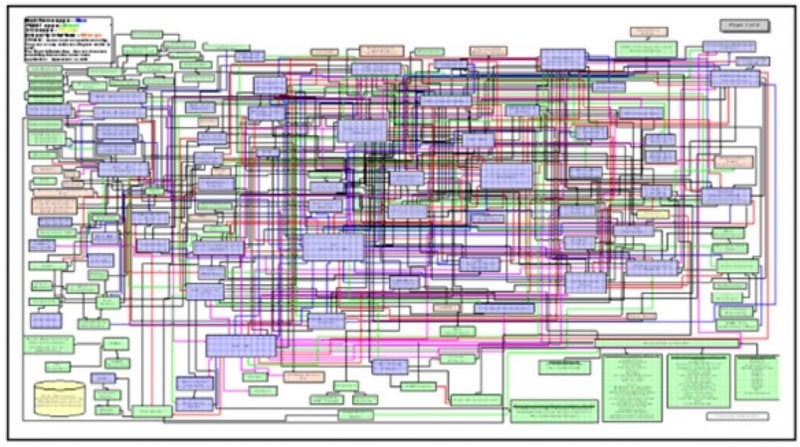
A typical clinical operating environment before unifying, according to Ed Leftin
He described Ora’s approach of bringing together CTMS, eTMF, and study start-up on one system with Veeva Vault Clinical Suite and shared tips on successfully transitioning to a unified clinical operating environment. Improved collaboration and real-time reporting and visibility were just some of the benefits cited.
The move from passive to active TMF management was high on the agenda for another year at Summit. In a passive TMF operating model, documents are only uploaded to the system when they are finalized. Conversely, active TMF management ensures all TMF information and processes are managed in the same system, in real-time, as they are being executed.
C.R. Bard, Inc and Daiichi Sankyo, Inc explored the advantages of transitioning from legacy systems to active TMF. Daiichi Sankyo, Inc’s Jamie Toth talked through their journey to streamline manual processes globally and the benefits achieved, such as improved quality control. C.R. Bard, Inc’s Rushil Sankpal shared his company’s dramatic increase in TMF maturity level – particularly with collaborative content creation and compliance and governance – since moving to Vault eTMF.
J&J Innovative Medicine’s Shayna Lambert suggested making inspection readiness part of a daily, active TMF routine. She led the audience step-by-step through preparation for a recent, successful inspection and offered a sneak peek at her inspection-ready checklist. She also reinforced how planning is vital for smooth-running inspections.
This year’s Summit was a great platform for peer learning and advice-sharing. It was exciting and informative to be part of those conversations. We’re looking forward to watching the industry’s progress over the next 12 months. Until next year…
To find out how unifying clinical operations can enhance your clinical trials, check out our unified clinical demo here.
Click here to catch up on this year’s Summit presentations