Industrywide Move to Purpose-built eTMF Applications
The industry has made significant progress modernizing trial processes by adopting purpose-built eTMF applications, which help increase visibility, oversight, and collaboration while driving a constant state of inspection readiness.
According to a recent industry-wide global survey, the use of eTMF applications has tripled since 2014. More than half (56%) of sponsors now use a purpose-built eTMF solution, versus just 17% five years ago.
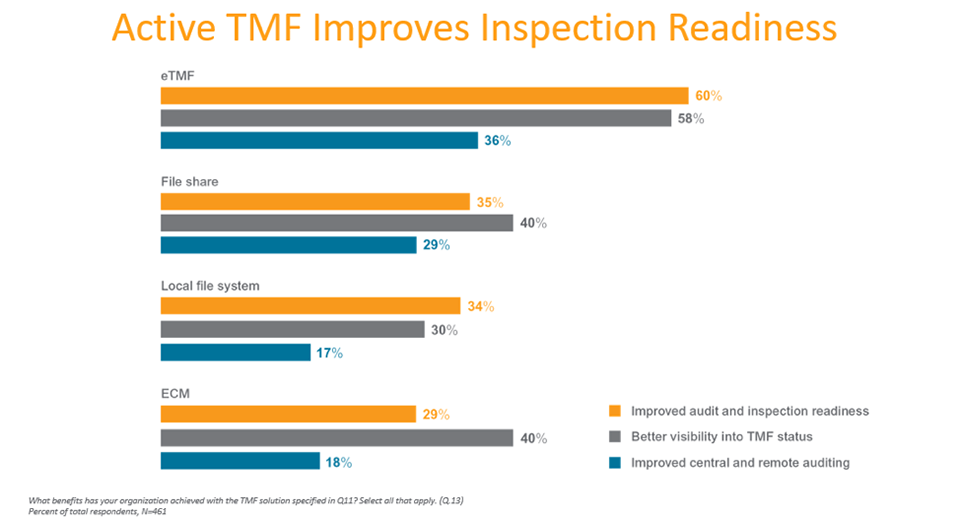
Those using purpose-built eTMF applications report fewer challenges with compliance and TMF tracking and reporting, and a positive impact on visibility, auditing, and inspection readiness.
Survey findings show purpose-built solutions are enabling more active, real-time TMF management and oversight.
Watch this short video to hear more findings from the Veeva 2019 Unified Clinical Operations Survey.