Blog
Addressing Complications with End of Study Media
Aug 26, 2021 | Doug Bain and Natalie Townsend
Aug 26, 2021 | Doug Bain and Natalie Townsend
 Guest authored by Doug Bain, Chief Technology Officer at KCR, an international full-service contract research organization (CRO)
Guest authored by Doug Bain, Chief Technology Officer at KCR, an international full-service contract research organization (CRO)
When it comes to archiving clinical data at the end of a trial, things haven’t improved in over a decade. Once data is locked, the administrivia begins. Data managers create PDFs for each subject casebook, save them to a physical storage device (such as a CD or DVD because thumb drives get expensive), create the corresponding documentation and instructions, and send everything to the sites for archiving.
That’s when things can start going wrong:
- The address to the site’s records department may be incorrect.
- The recipient may not confirm receipt as requested.
- The recipient may not check the content of the materials received as requested.
- The labelling and storage at the site may be incorrect.
- The site may not have means to read a CD or DVD.
- The encryption media and associated passwords may become separated.
In parallel, all PDFs must be manually uploaded to the sponsor’s eTMF. Files must be named, QC’d, and approved – the time to complete this can be measured in days and weeks for a typical study.
The list of potential problems is long, which makes tracking shipments and compliance a hassle, even for small trials. Now, imagine it for a trial with thousands of patients and 100+ sites. The production, tracking, and management of end of study media exhausts data management time and budgets and can impact the quality of closeout activities.
Veeva’s innovation to automate the end media distribution and filing to the TMF is one of the many reasons we at KCR are excited to begin working with Vault EDC.
Vault EDC provides a fully cloud enabled, validated, digital approach to data archiving. Workflows trigger the creation of CRF PDFs, sharing the PDFs with sites, and confirming receipt. Automating the process not only improves quality, it also reduces the effort required to speed site closure activities. Auto-tracking sites’ commitment to maintain the electronic files for the required duration also supports inspection readiness.
I’ll pass this blog post over to Natalie Townsend to share Veeva’s perspective.
 Thanks Doug, for guest-authoring this blog and for being such a great CRO partner. Addressing the problems around end of study media is part of Veeva’s strategy to modernize data management. Shipping physical media around by mail and manually tracking responses is just an old-fashioned way of working.
Thanks Doug, for guest-authoring this blog and for being such a great CRO partner. Addressing the problems around end of study media is part of Veeva’s strategy to modernize data management. Shipping physical media around by mail and manually tracking responses is just an old-fashioned way of working.
Veeva’s approach is based on four principles:
- Self-service — Sites, sponsors, and CROs can request and retrieve their own archives at any time, in human- and machine-readable formats.
- Automation – Once the study and site are closed, a workflow is initiated and sites are notified that documents are ready, so they can acknowledge receipt. Tracking is fully automated; there are no spreadsheets to update.
- Independent – Actions are completed without negatively impacting performance of studies.
- Media-free storage – sites can access their archives via download to their own physical or cloud archive or via SiteVault’s free cloud storage. There are no CDs, or other physical media that can get damaged or lost or become technically obsolete.
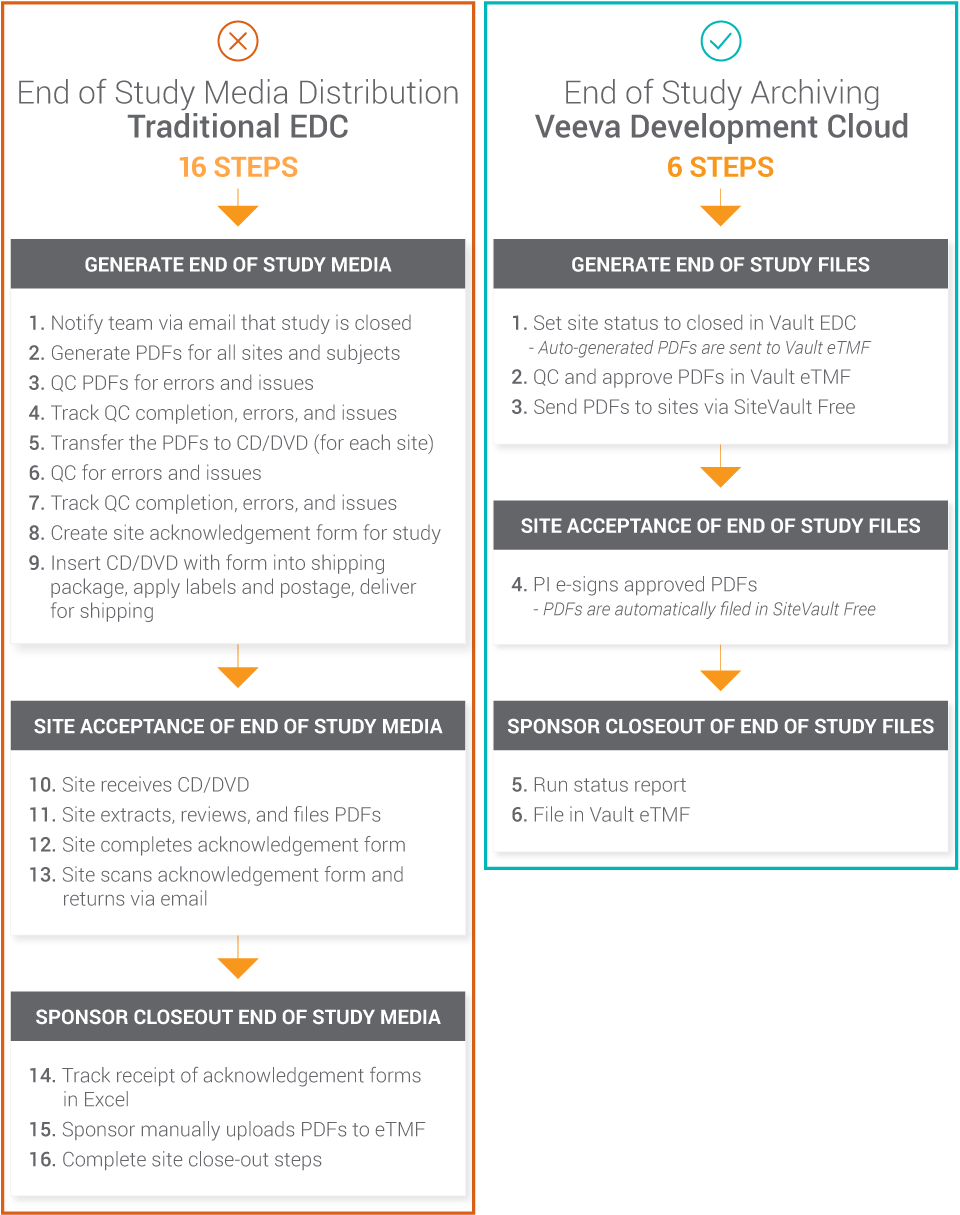
With Vault EDC, regulatory requirements are met, the burden of compliance is reduced, and the use of CDs is eliminated. When combining Vault CDMS with other clinical applications in the Development Cloud, manual disjointed steps in the traditional process can be reduced by over 50%, creating a much simpler and compliant workflow. The diagram below shows a 16-step process reduced to six steps, and the 16-step process is typically even longer because it doesn’t capture the repeated emails and outreach typically required when tracking shipments and compliance manually.
The automation provided by Vault EDC helps achieve compliance quickly and efficiently, with minimal administrative burden. And when connected with Vault eTMF and SiteVault, the speed and quality will be unbeatable.
Learn how Vault CDMS supports decision making in oncology trials.