Atrium Innovations Achieves Real-time Visibility Across Global Quality Operations
Reduced cycle times from compliant to action or closure by 50%
Streamlined interactions between quality, regulatory, and customer service teams
Enabled global sites to collaborate with real-time visibility
Delivering transparency for quality teams to reduce process times by 50% and improve collaboration with Veeva Vault Quality
The Challenge: Global Sites Working Separately
Atrium Innovations’ mission is to be a global leader in the development of vitamins, minerals, and supplements to promote healthy lifestyles. Its quality teams and internal contract manufacturing organizations (CMOs) had used paper-based systems to manage GxP controlled documents and quality processes. Many global sites worked independently with their own processes, standard operating procedures, and local copies of content and data. As a result, teams found it challenging to collaborate, improve key processes, and gain visibility into site activities.
“Atrium wanted complete transparency into each sites’ operations and challenges,” said James Huang, director of global quality systems at Atrium. “If all sites can access the same information, teams can collaborate to expedite resolutions and save time.”
“Veeva Quality Cloud transformed how Atrium works. We’ve propelled the company from working in siloes to a globally transparent environment where we collaborate seamlessly.” — James Huang, Director of Global Quality Systems
Global pharmaceutical companies are modernizing quality and content management in the cloud. With all processes, content, and data on a single platform, organizations can gain greater visibility into quality and make faster, more agile decisions. Cloud technology simplifies complex processes and gives geographically dispersed teams universal access to the same information.
“We wanted to adopt modern technology to harmonize processes and bring together all global parties for a more integrated and collaborative approach. Improving quality in the supply chain and accelerating processes demanded a new way to collaborate and manage quality,” said Huang.
The Solution: Veeva Quality Cloud
Atrium selected Veeva Quality Cloud, the industry’s first and only unified suite of quality applications for seamless, end-to-end quality and content management on a single cloud platform. Veeva Quality Cloud brings together Veeva QualityDocs for GxP document management and Veeva QMS for quality management, delivering one seamless user experience. With a unified quality management solution, Atrium improved its operational transparency, data security, and availability.
“Veeva Quality Cloud transformed how Atrium works,” said Huang. “We’ve propelled the company from working in siloes to a globally transparent environment where we collaborate seamlessly. We can include stakeholders across sites and invest the time we save into making our operations even better.”
Real-time Visibility for Timely Product Releases
CMOs’ batch records and supporting documentation are put together in real-time. Each department at Atrium uploads their records to a centralized location, making it easy to see the status of each batch. The CMOs also have immediate access to the certificate of analysis (COA) when it is available, from anywhere. This is a significant change from the previous process of collecting and assembling documentation for the batch record from different teams and waiting for a hard copy COA that could be damaged or delayed – impacting the timing of the product release.
“The batch record is automatically assembled in real-time, as documentation is available from the various teams. And once the COA is ready, the CMO can login from any location to print it. Real-time access to information in Veeva QualityDocs allows us to act and make decisions faster,” said Huang.
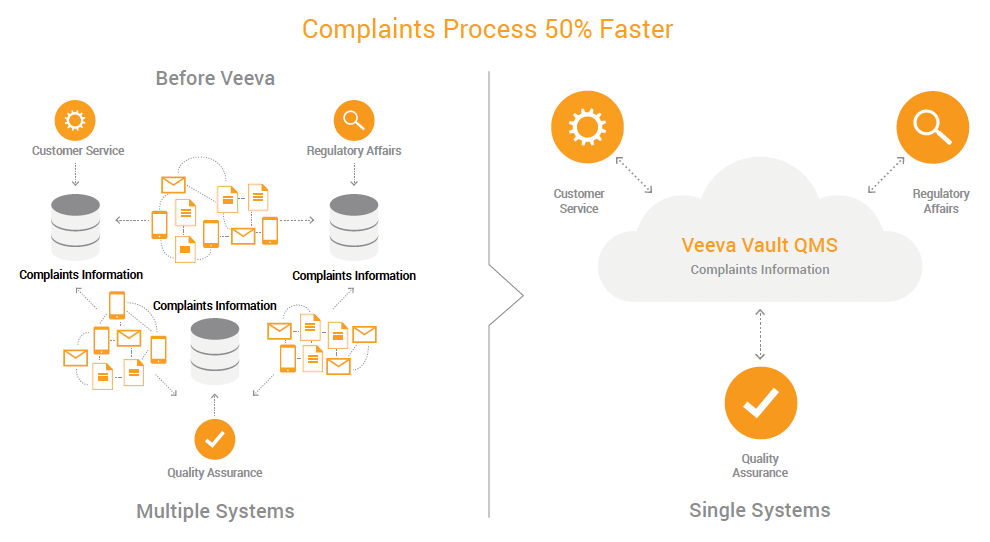
Seamless Complaints Processes for Accelerated Cycle Times
Quality assurance, regulatory affairs, and customer service departments at Atrium needed real-time visibility into complaints information. Before, the complaints team had to call regulatory affairs and quality, and then send the information over by email or phone. Each team would document the complaint in its own departmental system. The process of sharing complaint information and driving complaints to action or closure was inefficient, and often resulted in data duplication.
With a cloud-based QMS, all teams access the same complaints information and generate reports faster. The ability to share information across departments makes it easier for users to comply with regulatory requirements, and provides transparency into the complaints process.
“We’ve seen approximately a 50% reduction in cycle time from complaint to action or closure,” said Huang. “Regulatory affairs, customer service, and quality teams have complaints information at their fingertips with Veeva Vault QMS.”
Improved Deviations Management for Proactive Issue Resolution
Atrium’s team can drive efficient documentation of deviations and support global visibility and trends across sites with a cloud-based QMS. Trends such as recurring or unacceptable number of deviations can be easily identified. Atrium can also see if corrective and preventive actions (CAPAs) have been created to address the deviations, enabling the quality team to be more proactive in mitigating and resolving issues before they grow into complex challenges.
“Quality teams easily view the real-time status of deviations and associated CAPAs, identifying and tracking areas for improvement. With Veeva Quality Cloud, we have shifted Atrium’s quality function from reactive to proactive, which has positive impacts on the entire business,” said Huang.
Automated Reporting for Immediate Operational Insights
Dashboards and status reports give Atrium instant visibility into the health of their quality operations. KPIs track quality metrics, while operational reports monitor daily and weekly tactical metrics. Through this real-time capture of critical information, Atrium can be confident that it has an accurate view into each site and can aggregate the data for global visibility.
“Collecting and analyzing quality and operational metrics gives us complete visibility into global and site-by-site operations,” said Huang. “Since data is automatically collected, we can understand where delays occur and if they’ve been resolved. This gold mine of information in Veeva Quality Cloud means our quality teams can make better decisions with more complete data.”
“With Veeva Quality Cloud, we have shifted Atrium’s quality function from reactive to proactive, which has positive impacts on the entire business.” — James Huang, Director of Global Quality Systems
Efficient Audit Management
Atrium is managing and storing all audits in the new system to have historical and ongoing audit information in one place. Management will have global transparency into audit findings at each site and will be able to share critical findings or compliance risks with other sites to prevent or mitigate issues.
“Sharing critical findings with all sites allows us to quickly and collaboratively find the best approach to resolve issues,” said Huang.
What’s Next
To manage quality operations across global sites, Atrium took advantage of Veeva Vault Quality’s unified quality and content management platform. By harmonizing processes and collaborating across functional areas, the organization has accelerated workflows, improved processes, and gained real-time visibility into operational information to quickly resolve issues.
Looking ahead, Atrium aims to enable cross-functional processes that span quality and regulatory to streamline change management, such as having change actions in Veeva QMS translated to an event, commitment, or other action in the Veeva Vault RIM system. In addition, the organization plans to incorporate third parties into quality processes to enable greater collaboration.
“Connecting and unifying not only our QMS and content processes but also our regulatory information management with Veeva is very powerful. By enabling collaboration across sites and functional areas like quality, regulatory, and customer service, we can stay competitive in the market and continue to empower healthier lives,” said Huang.



