Blog
Focus Downstream to Meet Rising Clinical Expectations
Mar 07, 2023 | Sid Mohamoodally
Mar 07, 2023 | Sid Mohamoodally
“Over the past three years, record-shattering vaccine development timelines have raised expectations for clinical efficiency. Meeting them demands agility, and better ways to manage external data and prepare it for use downstream.” Sid Mohamoodally, Senior Director, Strategy, Vault CDMS, Veeva Systems
Clinical trial sponsors and CROs have seen, first-hand, what disruptive innovation can do. Not only have they kept clinical studies going despite COVID-19 restrictions, they have simplified processes, adopted new technologies, and improved efficiency, collaboration, and patient focus. The industry can never go back to the ‘old ways’ of managing trials and data.
But clinical professionals now face a paradox of rising expectations. Improvements made over the past three years have made faster cycle times a given throughout development. Month-long delays in study builds and lengthy interruptions for manual data cleaning and reconciliation are no longer acceptable, especially when changes must be made mid-trial.
The need to manage increasing volumes of patient data — most of it from external sources that cannot be managed directly in the EDC — has only added to the challenge. This data must be reported and analyzed by the biostatistics team and used in other key trial operations, emphasizing the need for a broader view of cross-functional stakeholder requirements. As the clinical environment continues to evolve, the shift from merely managing clinical data to understanding it within a wider, science-based context, promises to transform the data manager’s role and underscore its importance.
Clinical trial sponsors and CROs are already addressing top challenges in three key areas: reducing handoffs between clinical data and operations, a goal that Boehringer-Ingelheim is tackling in its OneMedicine program. They are improving access to clean patient data by adopting clinical workbench technologies and automation via artificial intelligence (AI), they are continuing to improve efforts to improve trial agility. Most importantly, they’re collaborating more effectively to ensure flexibility and a better experience for patients. Progress will be systematic and ongoing, and is already being seen.
Improving agility and reducing handoffs
Speeding study start-up continues to be a top priority. Some companies are shortening trial timelines by shifting the focal point for data management away from the EDC, which cannot store remote data from decentralized trials. Traditional EDC study builds have a mean cycle time of 69 days from protocol approval to database go-live, where agile approaches using a clinical data management system (CDMS) can reduce start-up times considerably. For Syneos Health, the move to an agile CDMS cut start-up times down to just a few weeks, according to Trevor Griffiths, director of clinical data management.
Also driving the shift from EDC to CDMS as the data-management hub is the fact that more studies are moving from the clinic to patients’ homes, resulting in a growing volume of patient data from wearables and other external sources that cannot be stored and managed directly in the EDC. In the past, the only way to incorporate this data was to take it offline and clean it manually — a costly, time-consuming, and tedious process. Some CDMS applications now feature clinical data workbench-type solutions, such as Veeva CDB, which allow external data to be cleaned and stored within the CDMS.
Combining the workbench with external connectors, such as data aggregation tools, analytics solutions, and pre-built integrations, maximizes flexibility for data management teams. “Externally, a connected approach addresses the issues of multiple sources for data, but working with different functions internally requires having a strong workbench,” said YaFang Wu, vice president of data management at Parexel. Combining the two approaches, Wu says, allows teams to manage different information and paths in one place.
Some companies are evaluating advanced approaches to help automate the process. Syneos Health, for example, is using AI to help its teams manage massive amounts of patient data. Efforts began with COVID-19 trials that involved tens of thousands of subjects. “With AI, we nearly eliminated manual review of medical data,” says Griffiths.
Connecting clinical data and trial management systems
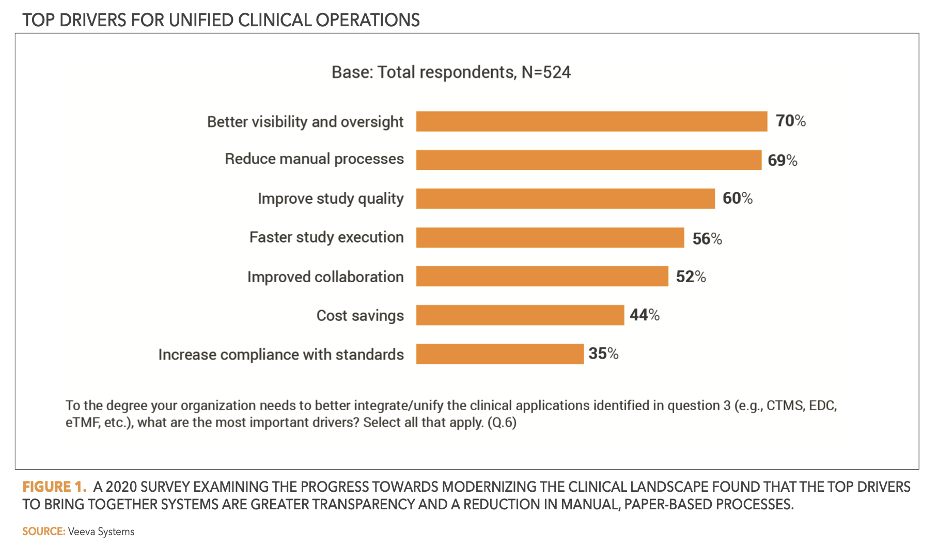
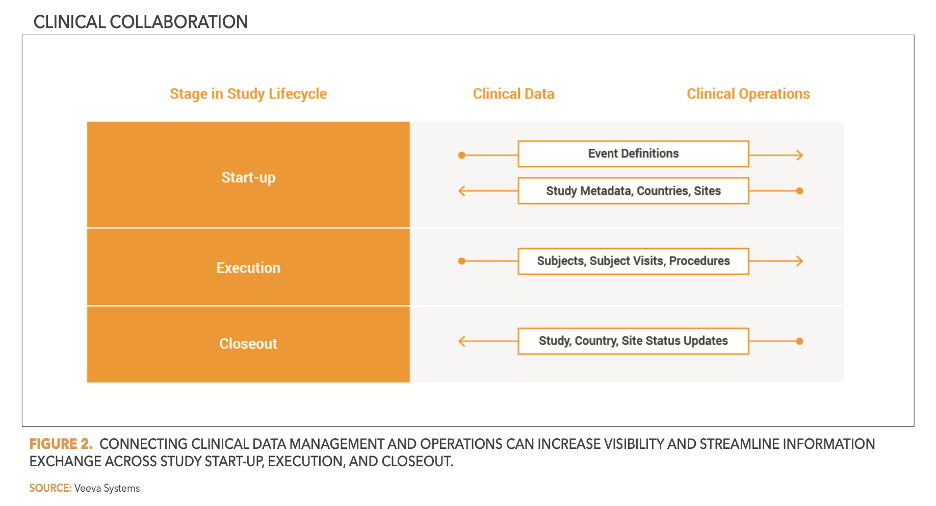
To enable automatic exchange of records and updates (Figure 1), companies are also working to connect clinical data and trial management systems. This approach allows sponsors and CROs to streamline remote monitoring (Figure 2) and reduce or eliminate data-entry errors.
Improved patient focus
As sponsors and CROs work to reduce the technology load for clinical research site partners, they are prioritizing the need to make digital clinical trials more patient-centric. Some are using eConsent or electronic patient-reported outcomes (ePRO) applications to enable patient consents and clinical data to be collected and recorded digitally, which simplifies data analysis, sharing, and reporting. Sam Ryan, director of data management at ICON, expects this trend to continue as clinical trials maximize patient convenience. In the process, the data manager’s role will change. “The explosion of data driven by decentralized approaches offers an opportunity to make roles more technical and data science-focused and to ensure that the right people are in place to set up, oversee, and manage each interconnection and data source in a trial,” he says.
Over the past three years, sponsors and CROs have raised the bar for clinical trial execution and data management. Meeting higher expectations for efficiency and connectivity will require ongoing innovation, if the industry is to reach the end goal of connected, patient-centric digital trials. The next stage of clinical innovation will be radically different from the last one, which was driven by necessity, but results already show what is possible.
Hear more stories about how companies are leveraging agile Vault EDC systems.