How Syneos Health is Speeding the Shift to Science-Based Data Management
“Testing new releases of Veeva EDC is completed in half the time required for other EDC systems.”
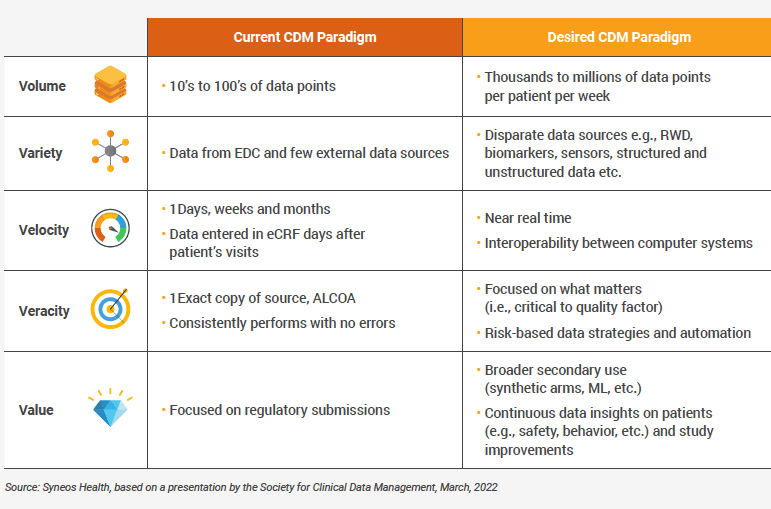
As clinical trials become larger and more complex, the growing focus on patient convenience has greatly increased the volume of decentralized patient data along with the need to reconcile it. Staying ahead of change requires close focus on the “five V’s” of Clinical data: volume, velocity, variety, veracity, and value.
Syneos Health, a leading fully integrated biopharmaceutical solutions organization, has approached these challenges by shifting from a traditional data management mindset to one based on data science (Table 1).
TABLE 1 – DRIVERS THAT WILL TRANSFORM CLINICAL DATA MANAGEMENT
INTO CLINICAL DATA SCIENCE
Over the past five years, Syneos Health has worked on more than 2,700 full-service clinical studies involving more than 743,000 trial patients and more than 90,000 clinical site partners. These include some of the largest, most complex trials to date, including those that enabled COVID-19 vaccines to be developed in record time.
Working closely with Veeva Systems, Syneos Health clinical leaders continue to focus on five key objectives:
- Improving internal agility
- Managing decentralized patient data more efficiently
- Increasing connection between clinical operations and clinical data
- Improving the user experience for internal and external stakeholders
- Taking a risk-based approach to clinical data management
A new role for clinical data management teams
“As innovation continues to shape clinical trials, clinical data management is emerging from its traditional industry role as a supportive back-office function to become integral to innovative product and service delivery,” says Nick Lakin, senior managing director at Syneos Health. New approaches to clinical data and use of a common data-management platform promise to increase agility.
This added flexibility will enable quicker response to market needs, allowing sponsors and CROs to shift effortlessly from functional service partnerships (FSPs) to full-service outsourcing (FSO) business models depending on each project’s needs. The new approach to data management will also facilitate the use of advanced technologies such as artificial intelligence (AI) and machine learning (ML).
Partnering with Veeva Systems, Syneos Health has developed the foundation needed for flexibility and integration. Based on the organization’s successful adoption of Veeva eTMF, clinical leaders decided to partner with Veeva to establish a modern foundation for clinical data management, planning to centralize data management by rolling out Veeva Clinical Data, including the platform’s clinical workbench, CDB, internally and with clients.
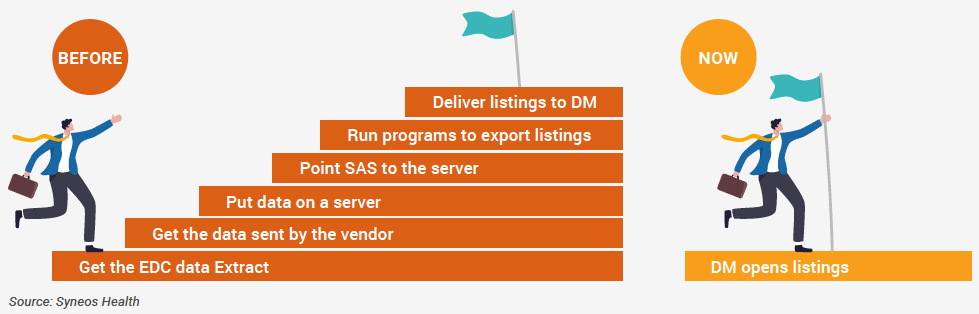
Lakin sees CDB, which reduces process steps by allowing remote patient data to be cleaned, stored, and processed centrally (Figure 1), as a key market differentiator for the CRO. Now that Syneos Health has gained experience using CDB with multiple clients, senior managers plan to establish a Center of Excellence for CDB within the company.
FIGURE 1 – WITH VEEVA CDB, SYNEOS HEALTH ELIMINATES
TIME-CONSUMING MANUAL DATA-RECONCILIATION STEPS
Significant gains in operational excellence
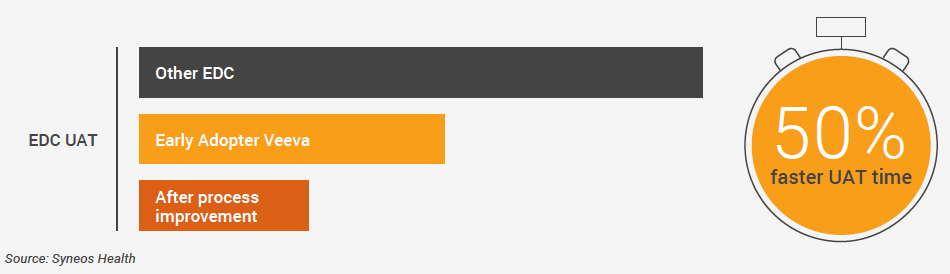
Improvements achieved with Veeva CDB add to the significant efficiency gains that Syneos Health has realized with Veeva EDC (Figure 2). During the UAT cycles prior to go-live and for amendments, Vault EDC reduces the testing burden to only what has changed and eliminates all the testing associated with custom functions.
FIGURE 2 – USING VAULT EDC, SYNEOS HEALTH HAS HALVED UAT TIMES
On a broader level, use of Veeva Clinical Data allows Syneos Health data management teams the ability to rethink processes and their design and make improvements as they integrate applications within the new platform. Having all clinical data in one place enables users to study trends and generate reports secure in the knowledge that all the information is clean, complete, and up to date.
Another major gain has been improved user experience — not just for sponsor or CRO data managers but for users at research sites and for monitors. Each type of user is assigned an interface that has been optimized for the work they do, whether data entry or verification. “Veeva Clinical Data makes the data management job easier,” says Lakin.
But achieving maximum value from Veeva’s platforms requires an agile approach and a long-term view. “Veeva EDC is not just an EDC replacement that you’d drop in,” emphasizes Trevor Griffiths, senior director of data management at Syneos Health. His team optimized results by viewing implementation as a journey of change, and a way to drive process improvement throughout the organization. Veeva has played a key role in driving positive change, says Lakin.
“Vault CDMS makes the data management job easier.” — Nick Lakin, senior managing director at Syneos Health
Best practices start at the planning stage
Succeeding with implementation requires clear focus, not only on change management and process optimization, but on end-to-end data flow. As Lakin puts it, “What would be the use of having the world’s fastest study build if you’ve only shifted the rate-determining step from one place to another?”
As with any significant process improvement, change management must be addressed thoughtfully when moving to Veeva Clinical Data. “You can’t just build the system and hope they will come,” Lakin says. “Expect resistance to change but consider carefully how to bring teams along with you on the journey.” He suggests that an effective communications strategy be developed concurrently with implementation and training plans. For Syneos Health, regular knowledge-sharing sessions from teams that had rolled out the application helped create momentum and faster user acceptance.
The company also facilitated change by creating a methodology for improving and standardizing processes and recruiting subject matter experts for an early adopter team to run through the proposed process changes. The team analyzed existing materials including SOPs and supporting templates, as well as standards, normal ranges, and how-to guides, then performed gap analyses to determine what enhancements were needed to reflect the new processes.
Updates and enhancements had been a gray area with past technology partners, but Veeva offered the team transparency on what would be possible and when. Each new release of Vault EDC delivered enhancements that further improved cycle times. “Testing new releases of Vault EDC is completed in half the time required for other EDC systems,” Lakin notes.
Veeva CDB also enables improvement by automating pre-processing and storing clinical data in a central location. The traditional approach to generating listings involved multiple steps. First, a data-management team would have to ask its EDC vendor to extract data. They’d then put that data on a server, run some SAS jobs, develop programs to create the listings, run those listings, and export them to data management. Using CDB and pre-processed data, teams simply open listings that have already been generated and appear in one place. They no longer need to transfer data between different applications. The application also sends messages to lab vendors and enables batch queues to be run automatically.
Business model flexibility and an entry point to advanced technologies
Having data in one place enables greater business model flexibility, allowing CROs to have one single data and resource pool so that they can use both FSP and FSO approaches to working with partners, shifting from one to the other depending on sponsor and business needs.
This approach not only improves consistency, but standardizes reporting models and provides comparable metrics for both FSO and FSP data. It also enhances skill sets, enabling CRO data management teams to execute both models easily. “You’re sharing best practices, enabling standardization and greater transparency. It also simplifies governance so you’re not burdening sponsors with multiple governance models,” says Lakin. “Providing one common platform for delivery by a sponsor or a CRO, in turn, enables the use of algorithms that can be deployed from one to the other,” Lakin explains. This includes the use of AI and ML to automate the process of checking for clinical data anomalies.
Currently, the industry uses manual methods to review and trend data to find outliers, creating a time-consuming bottleneck to greater efficiency. Using advanced technology, data managers could automatically find problems and confirm that they’ve been addressed or corrected. Syneos Health is exploring the possibilities for AI and ML in clinical data management with companies that include CluePoints and RxDataSciences. “Imagine a world in which two CROs or a CRO and a technology partner race to develop the best algorithms, and sponsors get to pick the best one and figure out how to deploy it. This is where things get exciting,” says Lakin.
These improvements, however, depend on a foundation of agility and accuracy. For Syneos Health, collaboration with Veeva and use of Veeva Clinical Data, featuring Veeva EDC and Veeva CDB, has helped the company’s clinical data teams tackle the five V’s (volume, velocity, variety, veracity, value) and respond more nimbly to change as clinical trials and data management continue to evolve.
In two video interviews and a podcast, Trevor Griffiths, senior director of clinical data management, shares insights into how the industry is moving from clinical data management to clinical data science, and how Syneos Health is advancing science-based clinical data management with Veeva EDC and Veeva CDB.


