Veeva Submissions Publishing
Reduce Rework and Delays with Continuous Publishing
Submissions Publishing generates electronic submissions for global health authorities. Veeva releases new templates and validation criteria to keep up with evolving regulations. Submissions Publishing leverages content plans created earlier in the lifecycle to start the publishing process as soon as individual documents are finalized.
Users can automatically create internal and external hyperlinks to connect references in the text. They publish submissions directly to health authorities from Vault, in markets where allowed.
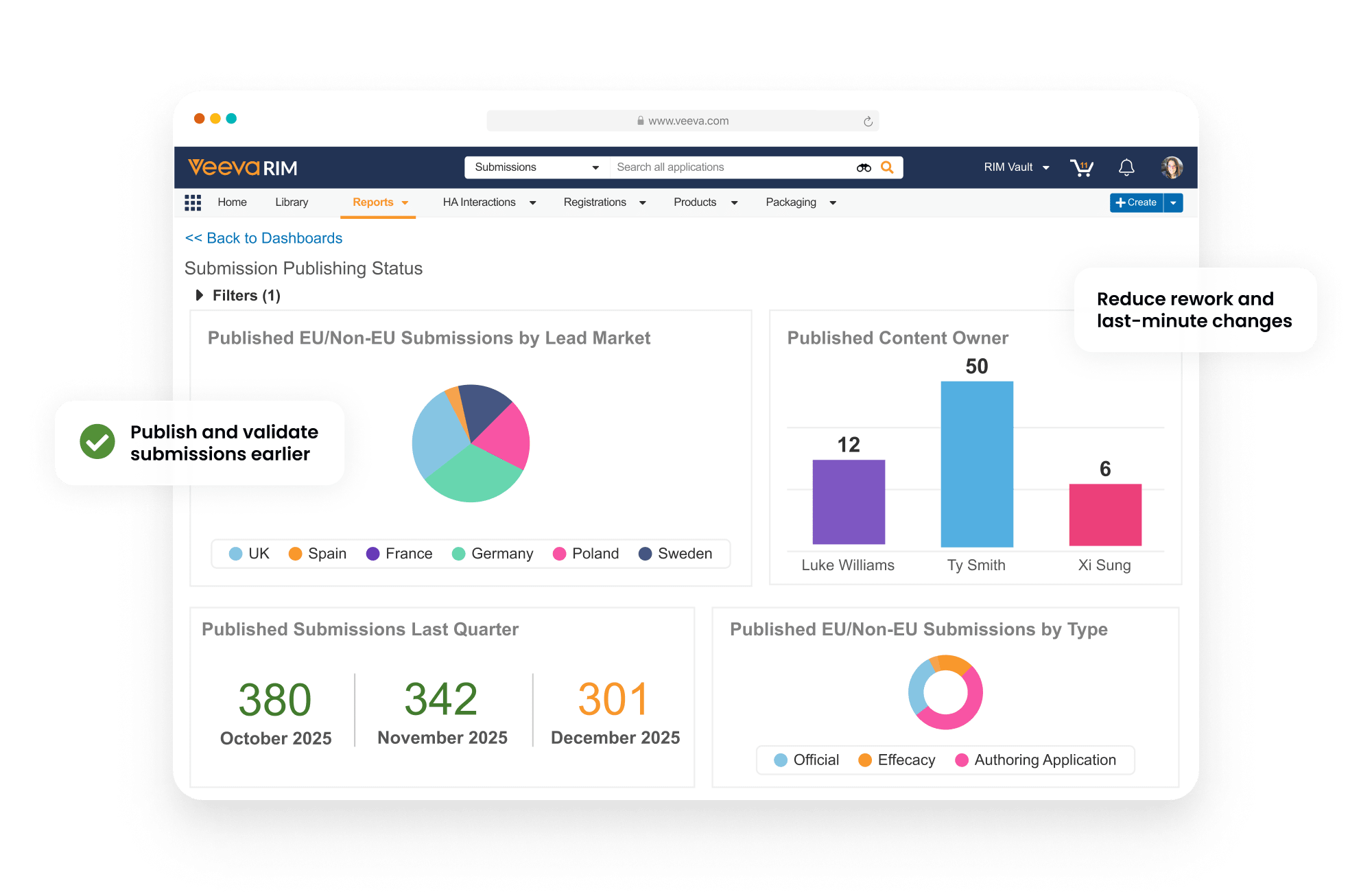
Dashboards and reports allow publishers to track each submission component as it progresses from authoring to completion.
Announced 2017 Status Mature Customers 51-100
To learn more, visit our continuous publishing resource hub
Overview
Reduce Rework and Delays with Continuous Publishing
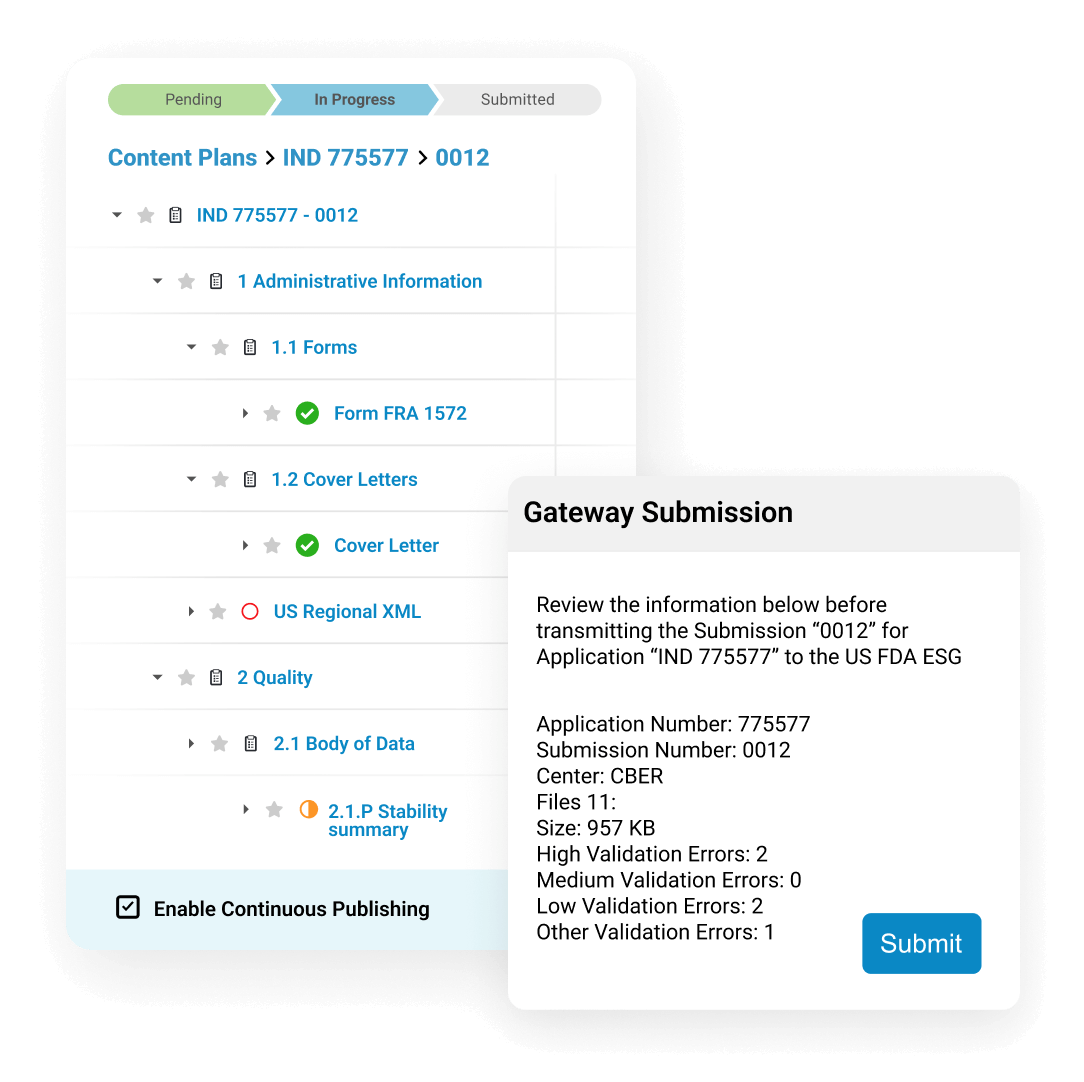
Submissions Publishing generates electronic submissions for global health authorities. Veeva releases new templates and validation criteria to keep up with evolving regulations. Submissions Publishing leverages content plans created earlier in the lifecycle to start the publishing process as soon as individual documents are finalized.
Users can automatically create internal and external hyperlinks to connect references in the text. They publish submissions directly to health authorities from Vault, in markets where allowed.
Dashboards and reports allow publishers to track each submission component as it progresses from authoring to completion.
Impact
Less time, less effort
50%
reduction in submission creation time
5 weeks
shaved from NDA
timeline
700 hours
saved with direct gateway submissions
Why Veeva Submissions Publishing
Reduce rework and delays
Customer Success
Veeva RIM is trusted by 400+ top
and emerging biopharmas