Blog
Strategies for Navigating Global Regulatory Change
Nov 05, 2024 | Paul Attridge
Nov 05, 2024 | Paul Attridge
In a rapidly changing regulatory environment, biopharmas are pressured to efficiently manage large amounts of regulated data and documents. To navigate these challenges, leaders are consolidating systems into a unified regulatory information management solution to increase cross-team collaboration and provide end-to-end visibility. With a simplified environment, companies are more agile and can better comply with a complex, global regulatory landscape.
Sustainable change management programs are essential to support the growing data requirements of regulations like ePI and IDMP, which involve 50-60% more data than xEVMPD. Critical for navigating EMA’s phased implementation of IDMP standards and ongoing eCTD 4.0 changes is an agile change management strategy.
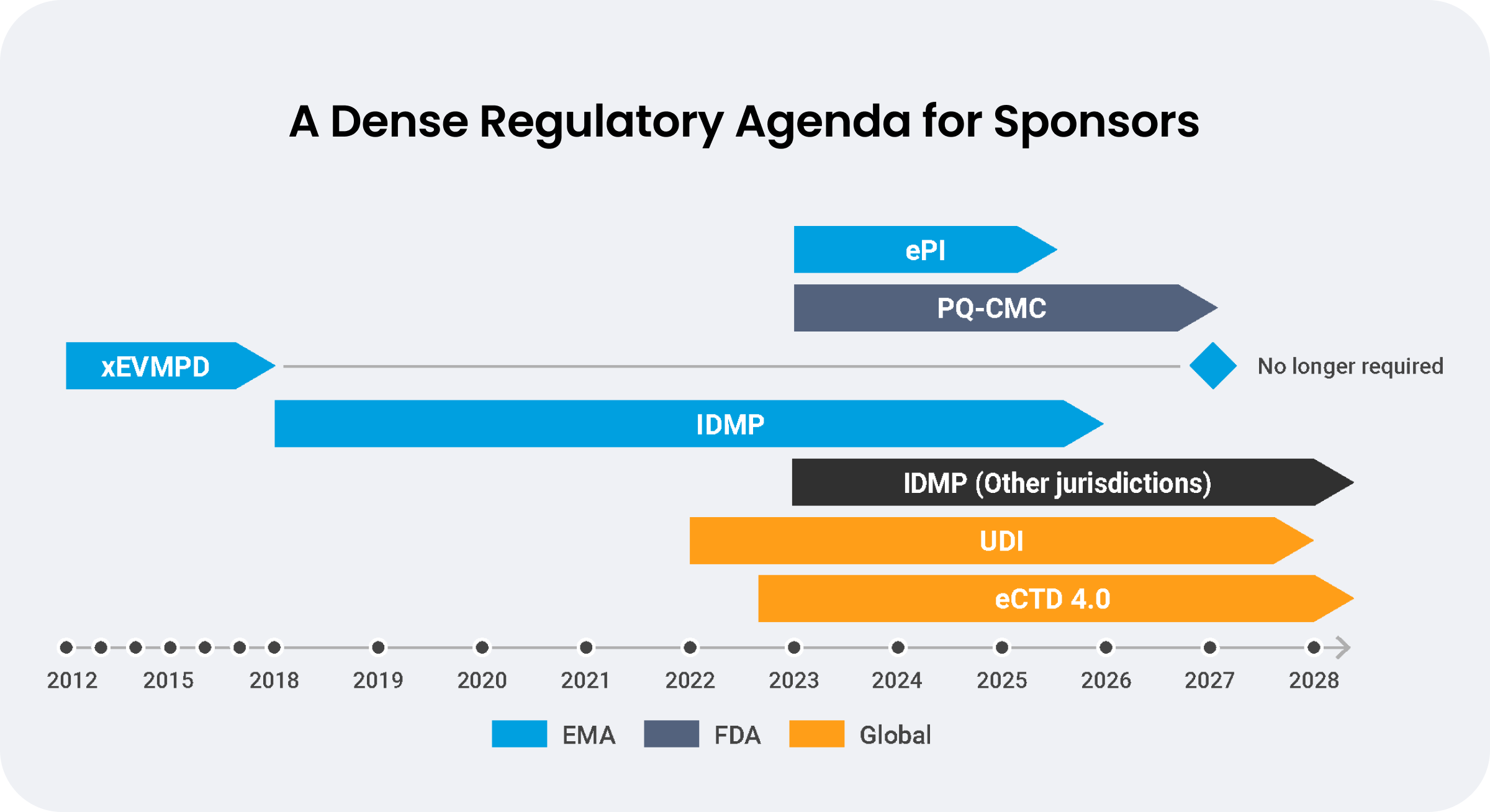
Source: Veeva Systems
Traditional regulatory change management strategies rely on distinct information systems for each new requirement. However, the volume of impacted data and documents has made point solutions unsustainable. Three industry leaders share how they are preparing for rapid global regulatory changes using a unified RIM solution to enable better data quality, automated updates, and end-to-end visibility.
Nordic Pharma streamlines RIM for compliance
Nordic Pharma adopted unified RIM to consolidate its fragmented systems, for faster, more efficient regulatory processes. With automated updates on a single cloud-based platform, the company can maintain regulatory compliance with reduced overhead.
The phased implementation began in 2022 with Vault Registrations and Vault Submissions, enhancing document management and analytics. By 2024, Vault Submissions Publishing was added to accelerate submission timelines through improved control, visibility, and validation. With a continuous publishing process on a unified platform, Nordic Pharma can now review and validate in parallel, reducing errors and rework, and staying current with regulatory changes.
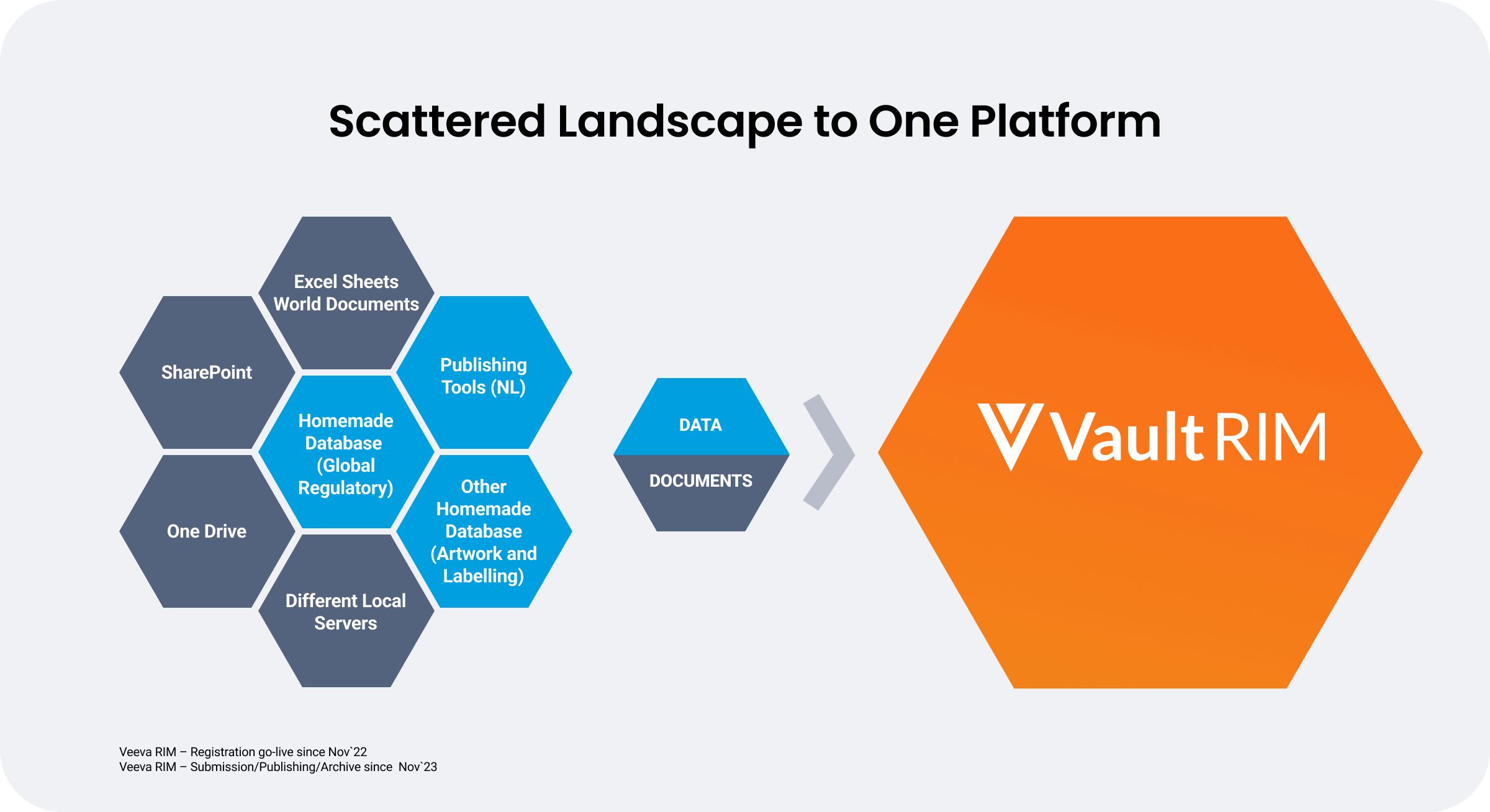
Phased regulatory transformation strategies
One global biopharma had an end goal of enhancing overall efficiency and transparency in data management for real-time insight and decision-making. Beginning a multi-year digital transformation in 2022, the organization started with a re-evaluation of business objectives to meet desired outcomes. This included defining clear roles and responsibilities, reducing manual tasks, eliminating duplicate data management, and consolidating disparate regulatory systems for enhanced visibility.
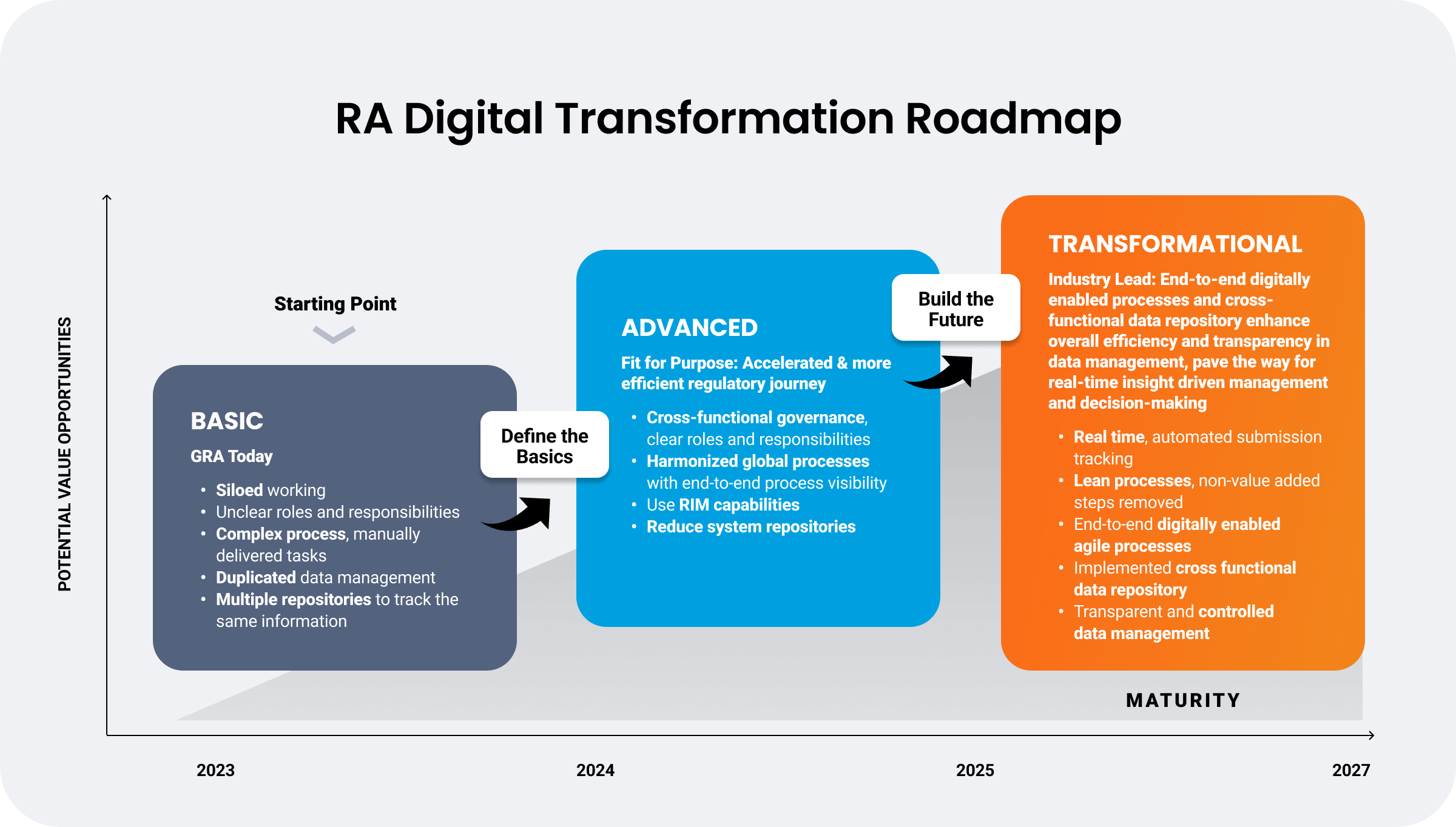
Now in its second phase of transformation, the company is already making progress — improving cross-functional collaboration, enhancing global visibility, and reducing system repositories.
The biopharma’s final phase of regulatory transformation will focus on end-to-end submission visibility, cross-functional data connections, and more efficient regulatory operations. With a unified RIM solution, they will enable real-time data-driven decision-making for an agile approach to global regulatory change management.
Efficient regulatory change management
Transforming regulatory operations can impact current ways of working and proper change management is essential for adoption. One global biopharma’s strategies included securing stakeholder buy-in, fostering cross-functional collaboration, training subject matter experts, and closely auditing migration processes.
A tailored organizational change management (OCM) framework helps prioritize initiatives based on potential impact and scope. Regulatory teams can determine the necessary level of change intervention through a three-step approach:
- Define the change: Identify key factors such as the type, scope, and rationale for the change.
- Qualify the change: Score the change using a matrix of predefined parameters (e.g., business impact, implementation complexity) to categorize the level of change.
- Develop a tailored change strategy: Provide communication, training, and guidance proportionate to the impact on user groups.
Successful regulatory transformation requires a tailored and iterative OCM approach to ensure adoption and sustainability. Clear goals and preparation are essential for maximizing the value of new technologies.
Continuous improvement with unified RIM
By adopting a “change as usual” mindset, supporting cross-functional collaboration, and successfully leveraging technology, regulatory operations can more easily stay current with changing global requirements. With Veeva Vault RIM, companies gain end-to-end visibility and automated updates, making it easier to adapt to industry changes.
Regulatory transformation is a continuous improvement process. Unified RIM not only helps companies meet today’s health authority guidelines, but also positions them to innovate at scale as the regulatory environment grows more complex.
Explore Pharma’s Regulatory Checklist, and prepare for the new regulatory agenda with Veeva Vault RIM.