AstraZeneca Optimizes Study Start-up with Data-Driven Strategies
40
Countries live with
Veeva Study Startup
450
Hours saved in one year on site enrollment readiness
260
Working days eliminated for monitors with workflows
Large biopharmaceutical company AstraZeneca has three key study start-up goals: accelerate site activation, eliminate manual tracking and planning, and minimize email-based communication. Managing study start-up activities across multiple geographies is no easy task for large companies like AstraZeneca. Operations teams must account for each country’s specific requirements and manage international regulations, often while working in silos.
Anna Wenelska was brought in as Veeva Clinical Vault Study Startup product owner to help AstraZeneca unify its processes with a centralized system. Because of her experience using Veeva eTMF, Wenelska was well-positioned to help AstraZeneca unify its processes with a centralized system, adding Veeva CTMS and Veeva Study Startup. The company’s study teams have saved thousands of hours through these system enhancements. “These solutions provide up-to-date site data and personnel lists in the system, enhancing the completeness and timeliness of data entry at an early stage of the study,” comments Wenelska.
A need for unification
AstraZeneca introduced Veeva eTMF in 2015. “This is how I came to know Veeva,” Wenelska says, adding: “Our people loved the system compared with our previous TMF. It’s really user-friendly and fast, allowing us to do bulk uploads.”
However, the company did not have a global tool or process supporting study start-up. Without a system in place, siloed information made it challenging to accelerate study start-up activities in line with business goals.
In 2018, the need for a new CTMS catalyzed the decision to implement Vault Study Startup in parallel with Veeva CTMS, adding to the team’s existing Vault eTMF. Wenelska was appointed study start-up product owner and with the help and expertise of process owners and study teams, began searching for a solution. “Our study teams know their country’s regulations, so it was a journey where we learned from each other,” she comments.
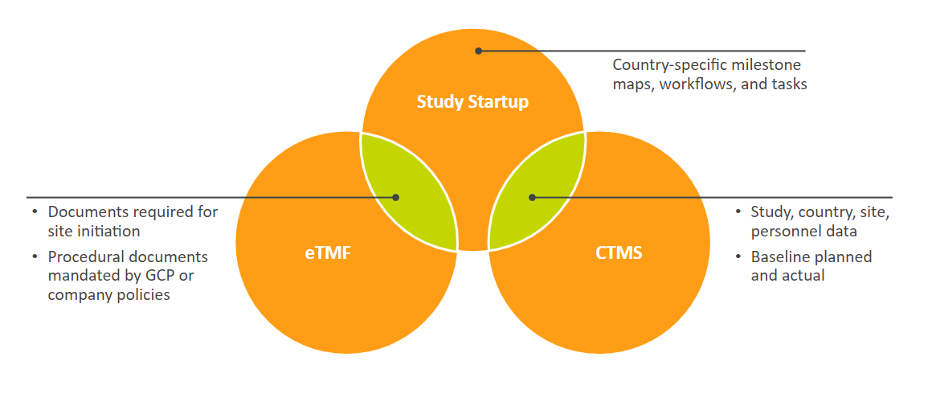
Users can create content inside the eTMF and collaboratively amend it, while preparing submission packages for start-up. “If you have all the components, I think that’s where you get the most value,” comments Wenelska.
“If you have all the components [of Veeva Clinical Platform], I think that’s where you get the most value.” – Anna Wenelska, Veeva Clinical Platform Study Startup Product Owner, AstraZeneca
Going live with a ‘big bang’
The study start-up team implemented Veeva Study Startup in seven key countries initially, with close support from Veeva. Her team undertook a detailed mapping exercise to understand key processes and standardize them. This then acted as a launchpad for another 23 countries, and a further 10 that followed after initial deployment.
Wenelska explains why she chose the a ‘big bang’ method of implementation: “We really wanted a fast, modern solution to be adopted by everyone, which reduces manual tracking, planning, and communication.”
She adds: “We could have started slower, launching a pilot with a few countries before adding others. But on the other hand, a big bang approach means that every country could immediately benefit from the solution.”
The implementation team could standardize and implement process improvements for all study teams, and each country could use the Veeva Hypercare period to build a community of best practices.
As with any new system, there were some key learnings. Wenelska found it time-consuming to collect the country-specific requirements and manage change requests. Providing tailored training for all countries was also a challenge, as she only had a view of what was not applicable for each country, rather than what was.
AstraZeneca experienced several advantages of using agile methodology to launch Veeva Study Startup. The agile methodology required a significant mindset shift for teams but gave Wenelska a yardstick for future continuous improvement. Wenelska summarizes her recommendations to other companies looking to implement a study start-up solution: “Regardless of the size of your team, plan your resources for Veeva Hypercare. I’d also recommend making data accessible and transparent. This will help both study teams and technology teams manage the systems.”
Reaping the rewards of continuous improvement
From day one, Wenelska has championed a clear approach to continuous improvement. She reviews Veeva’s new Veeva Clinical Operations features regularly, including non-auto-on features, to deliver constant value and time savings to users.
AstraZeneca teams capitalize on the Veeva Study Startup checklist survey feature to confirm a site’s readiness to enroll patients. Previously, users would need to download the most recent version of the form, populate it, load it to DocuSign to get an eSignature, then upload it to the eTMF. But Wenelska realized that, given it was an internal document, an eSignature wasn’t required. She encouraged teams to move to the checklist format, eliminating the redundant processes and replacing them with review and approval workflows embedded in the checklist. This proved to be a key enabler of operational efficiency. “It’s better to have a data format that is integrated into the eTMF and fully reportable,” says Wenelska. “In the last year we saved 450 hours of local study team time just by doing this,” she explains.
Based on the success of the checklist feature, AstraZeneca wanted to introduce a method to centralize the site quality risk assessment process and speed up the review and approval workflow for local study managers. The centralized monitoring system analyzes site data and assigns risk scores to help monitors plan their activities when starting work with a new site. In the old process, the centralized monitoring system provided a downloadable document with the risk score. A clinical research associate (CRA) would download and evaluate it, before providing a final score that would have to be approved and signed by the local study manager before it was uploaded to Veeva eTMF.
By building an integration between the centralized monitoring system and Veeva Clinical Platform, CRAs can review information and get decision approval in one location, speeding up the workflow. This process saved a total of 260 working days of monitors’ time in the last year.
“In the last year we saved 260 working days of monitors’ time.” – Anna Wenelska, Veeva Clinical Platform Study Startup Product Owner, AstraZeneca
AstraZeneca delivers improvements like these every year. Wenelska summarizes their approach: “We evaluate what teams are doing manually. If they’re using multiple trackers, or if people are using similar trackers, then you can find the most value and move the task to the system. And once it’s in the system, you can implement data-driven strategies for improvement and collect feedback.”
Keeping a finger on the pulse
Operating in 40 countries means there are numerous study start-up methods across AstraZeneca. A top priority for the company was honoring the specific study start-up workflow requirements of each country, while achieving user alignment and consistent use of the tool. Measuring adoption of Veeva Study Startup was critical to achieving this goal. Usage analysis revealed that not all teams were entering their requirements in the system.
Wenelska explains her reaction: “We realized we needed to establish some minimum requirements, but have templates that still allow for differences, as this is the whole advantage of using Veeva Study Startup. We still honor the country-specific requirements but give them a baseline to follow.”
This improves efficiency and consistency. As users follow the template for one study, subsequent studies are easier and faster to set up.
With all this data in place to monitor how the tool is being used, Wenelska can check whether teams are using Vault Study Startup to assign tasks, create new ones, replace meeting notes with tasks, and divide activities. “By reviewing this data, you can see where any problems might be and which teams you should address them with,” she explains.
A recipe for joint success
AstraZeneca and Veeva have collaborated closely to optimize Veeva Study Startup globally. For instance, when one user flagged that their team needed to be able to repeat set-up settings across all sites for every study start-up, Wenelska’s team reviewed the current features and shared this with Veeva to address.
“It’s really worth collaborating with Veeva. We are communicating the needs that arise on our user’s end, and often we are able to adopt some features thanks to that.” – Anna Wenelska, Veeva Clinical Platform Study Startup Product Owner, AstraZeneca
Using the reference site feature, the team moved set-up settings from a single instance to all instances in just two clicks. “We share this information with Veeva and they respond to our needs,” explains Wenelska.
She concludes: “Active collaboration with solution providers like Veeva and with industry peers is an important success factor. Our success is their success.”
Learn more about eliminating manual start-up activities with Veeva Study Startup



