Blog
Streamlining Biopharma Content Review with a Tiered Approach
Dec 30, 2024 | Pamela Bonanno
Dec 30, 2024 | Pamela Bonanno
Rising demand for personalized and readily available commercial content drove creation 7% higher globally in 2023 compared with the prior year. Despite the volume increase and industrywide demands on content teams to expedite the delivery of compliant material, many biopharmas haven’t updated review and approval processes.
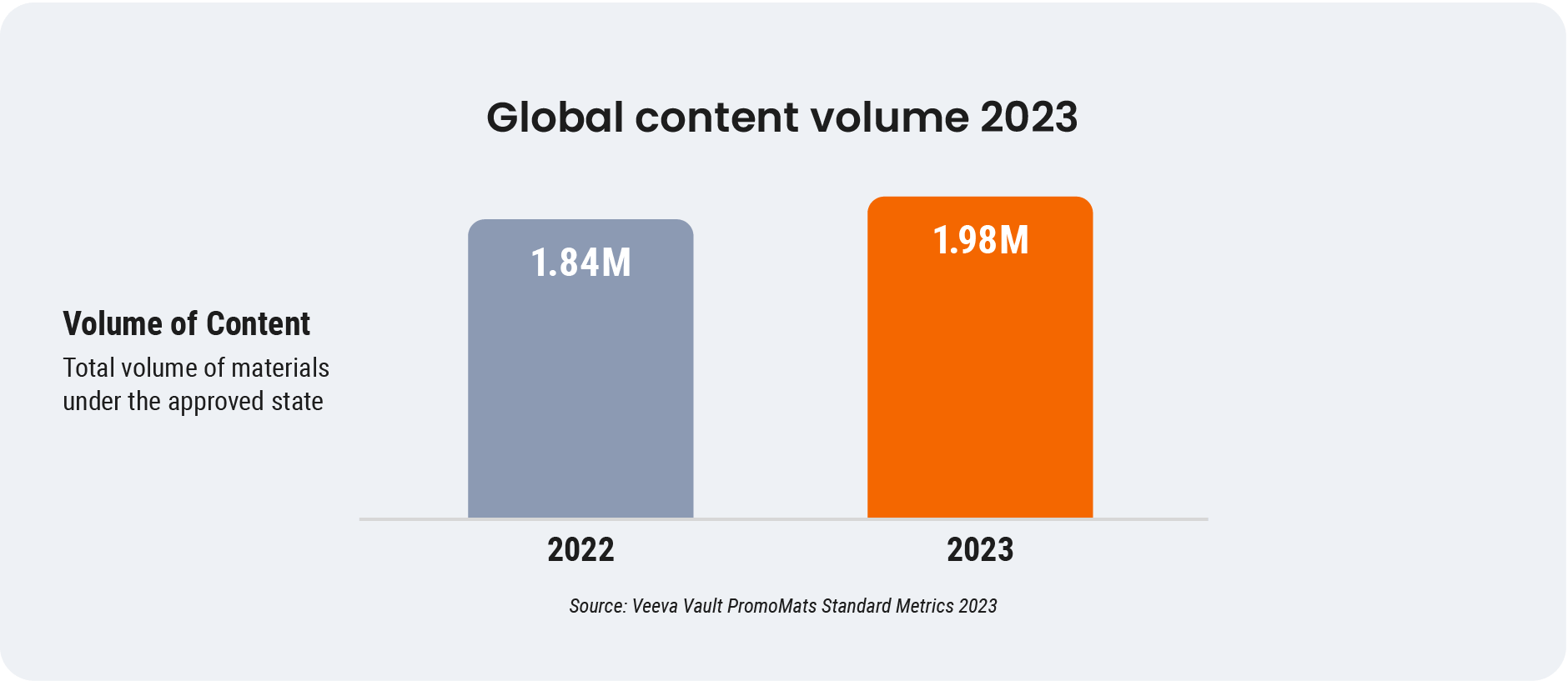
Regularly revisiting and refining your content approval processes is essential to maintaining effectiveness and efficiency for all stakeholders. A tiered approach capitalizes on your teams’ capabilities and delivers on healthcare professionals’ (HCPs’) content needs. Perhaps its greatest value is the ability to move a larger percentage of your content through streamlined tiers compliantly and efficiently, freeing resources to focus on high-value, high-risk material.
The need for a tier-based review process
Content approval is a complex and delicate process that can create risk if not executed well. Teams should exercise caution when modifying their approval activities to minimize disruptions. However, systems become more complicated as you add content and introduce regional variations.
A common default in approval processes is to apply an identical approach to all content, regardless of complexity or compliance risk. A one-size-fits-all approach creates unnecessary delays, which are problematic at any time but become acute when tied to a campaign. For example, your customers expect immediate access to material when you present new clinical data at a conference. Delays caused by inefficient review and approval processes can result in missed opportunities, as customers will seek information elsewhere.
A tier-based approval system categorizes content by risk level, assigning various review processes and limiting the roles involved based on the complexity and risk of each content piece. The model provides strategic benefits across functions:
Increased efficiency and better resource allocation: Reviewers spend less time on low-risk content, allowing them to focus on high-risk materials that require more thorough examination. Prioritization leads to faster overall approval times and enhances the quality of the review process for MLR.
 Brand managers
Brand managers
Faster time to market and greater scalability: Tier-based review enables brand managers to publish content faster, a boon for handling time-sensitive or derivative materials (e.g., following a conference). Flexibility improves responsiveness to market demands while allowing for easier adaptation and faster content reuse.
 Content creators
Content creators
Improved collaboration and continuous improvement: Streamlined processes help content creators collaborate more effectively with brand teams, fostering an environment of continuous improvement. Coordination can enhance overall productivity over time.
Key considerations for implementing tier-based review
If you’re considering a tier-based model, address foundational elements in three core areas:
- Regional variation: Regional differences often complicate content approvals. To the extent possible, consolidate your approval models across regions. This step will ensure that you apply tier-based logic consistently while allowing for necessary local adjustments.
- Content type and intent: Before teams can assign content to the correct tier and review path, they must be able to distinguish between content types and the intended use of the data or documents (e.g., promotional vs. non-promotional). As a result, it’s critical to establish and enforce standard definitions for content types and purposes.
- Modules and reuse: Using pre-approved or modular content templates helps structure content in a way that works better in the review process, reducing the need for full reviews of every piece. Modular content coupled with tiered reviews compounds the value biopharmas gain because MLR gains fast familiarity with templates and can easily spot aberrations.
Start streamlining approvals with a leaner process
Implementing a tier-based content review process requires careful planning, alignment across teams, and change management. Follow three foundational steps to begin:
- Gain clear alignment on taxonomy and metadata structure: Establish a well-defined taxonomy and metadata structure to support the tiered review process. Review and refine your existing content categories to ensure they accurately capture key attributes, such as intended use and target audience. Alignment is crucial for defining risk levels or tiers and requires coordination with all relevant stakeholders.
- Define logic attributes and tiers: Identify the key characteristics that determine how teams will categorize documents and data into the review tiers. Consider material intent, target audience, and content type (e.g., derivative vs. non-derivative). Create a map or decision tree to clarify how the attributes influence tier assignments and ensure you have stakeholder consensus on the final criteria.
- Assess functional needs across markets: While having a global framework for tier-based review is important, consider local market needs as well. Collaborate with teams across markets to ensure the system meets local regulatory needs without adding unnecessary complexity. Adaptability allows for the inclusion of additional reviewers when necessary, ensuring the process remains relevant to specific market conditions.
Shaping the future of improved content reviews
From brand introductions to HCP-rep meetings, tailor-made content makes customer touchpoints more impactful. Today’s MLR and content teams, including agency partners, are instrumental in shaping the future of individual and collective efforts to improve the content approval process.
With streamlined, tier-based reviews supported by Veeva PromoMats, teams can collaborate and produce content more efficiently and in compliance with standards.
Ready to develop a tiered approach to content approval processes and boost your team’s effectiveness?