CG Oncology Modernizes Trial
Management Approach
Brought CTMS in-house with a lean team of nine clinical and two IT employees
Centralized planning, performance tracking, oversight, and trial milestones
Cut down on manual activities and streamlined its clinical ecosystem
For many growing biotechs, it can be difficult to choose a clinical trial operating model that works for the organization’s size, budget, and case volume. Some biotechs see in-house trial management as their end goal. Others may outsource most or all their trial activities to a CRO. But, with a modern, cloud-based clinical trial management system (CTMS), companies can maintain operational oversight regardless of their model.
Dealing with fragmented and manual processes led John McAdory, Vice President of Clinical Operations at CG Oncology, and his lean team of 11 employees to implement a new in-house CTMS. “When I first started at CG Oncology, we were building our studies from scratch each time. We had a CTMS, but relied mostly on our CRO to collect information like action items, deviations, and monitoring reports without relying on spreadsheets,” he explains. “But, as our pipeline grew, we realized we needed a more robust system that gives access to our data in real-time. From an oversight standpoint, we wanted to make sure we were in compliance because, as a sponsor, we’re responsible for trial conduct. If you’re tied to a CRO and you’re using their systems, you lose the system if you leave the CRO.”
Selecting the right CTMS
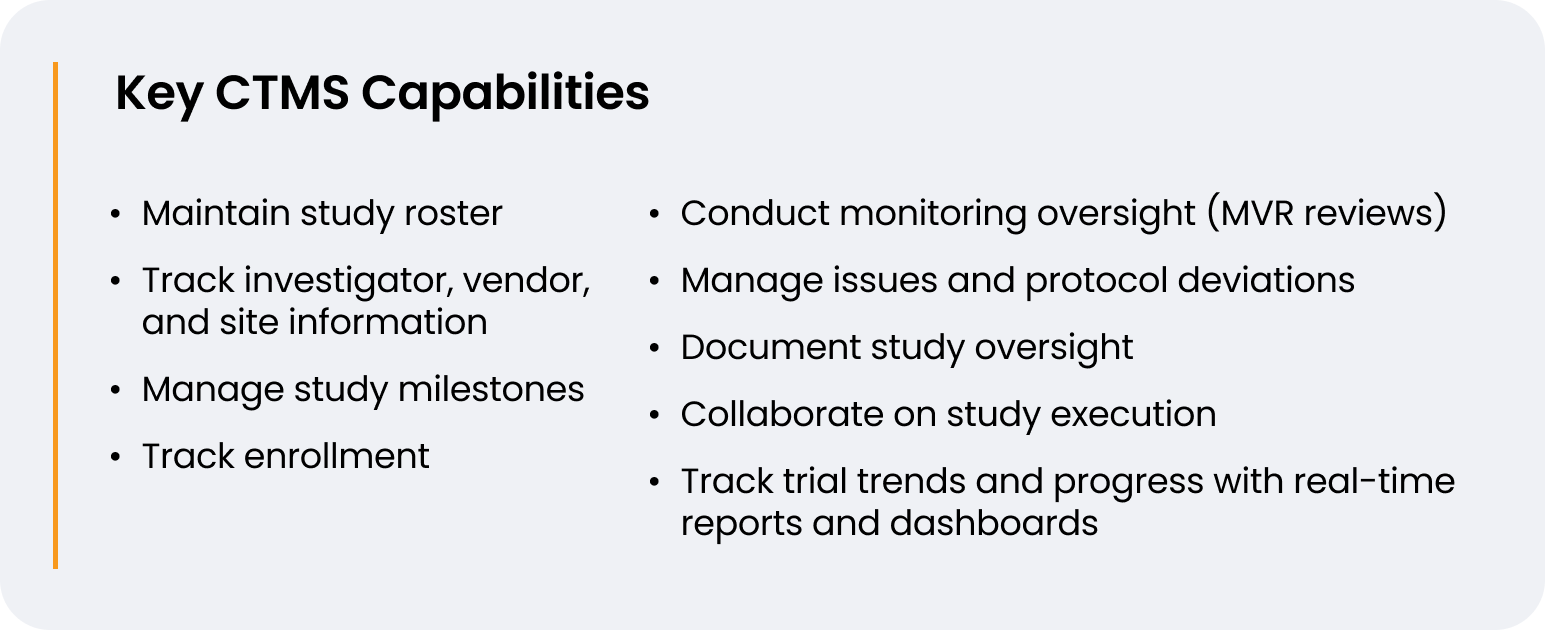
When choosing a CTMS, McAdory wanted a full-featured solution that would evolve as CG Oncology grew its trial portfolio. “I was looking for efficiencies and Veeva CTMS met all my requirements. Not only is it a centralized repository for trial management, it also has built-in analytics and dashboards. I can create my entire site list in CTMS, track how things are trending, and build customized reports,” he says.
Although data migrations can be daunting, especially for smaller clinical teams, McAdory found the process to be quite smooth. “We worked with our implementation team at Veeva to map information from our legacy system into Veeva CTMS,” he says. “When you’re doing data transfers, you’re always worried that something might go wrong. But, going through the mapping process made it a lot easier.”
Why every biopharma needs a CTMS
Veeva CTMS helps CG Oncology centralize planning, performance tracking, subject information, deadlines, oversight, and trial milestones. Now, McAdory’s team has an auditable and reportable record of oversight activities to satisfy regulatory requirements. “Having a CTMS is a great advantage for us during inspections. We can easily show the FDA that we have a validated system and that we’re in compliance with federal regulations,” says McAdory.
“Having a CTMS is a great advantage for us during inspections. We can easily show the FDA that we have a validated system and that we’re in compliance with federal regulations.” – John McAdory, Vice President of Clinical Operations, CG Oncology
McAdory advises biopharmas against relying on spreadsheets or documents for study tracking, even if they outsource most of their trial portfolios. “If you’re not using a validated system and you get inspected, that’s not going to fly with the FDA. The first thing they’re going to ask is ‘let me see your validation documents and what systems you’re using,’” he explains. “If you have your own CTMS, you can capture everything from monitoring reports and metrics to protocol deviations to give you better oversight of your studies.”
“If you have your own CTMS, you can capture everything from monitoring reports and metrics to protocol deviations to give you better oversight of your studies.” – John McAdory, Vice President of Clinical Operations, CG Oncology
Improved oversight, improved outcomes
With Veeva CTMS, McAdory’s team works faster and more efficiently with real-time access to trial data. “Before, we were keeping spreadsheets and dashboards in Excel. We were never sure: is the data real-time? If you update the report on Friday, did the data change by Monday when you sent it out?” he remembers. “Now, we can go into our CTMS at any time and trust that the information is accurate.”
Now that CG Oncology has modernized its CTMS, the company is looking beyond trial management toward a connected clinical ecosystem. “We’re seeing the benefits of a platform approach across the entire company. It’s really changing the way we work.”
Learn more about how a CTMS can help you take control of your trials.


