Industry Challenges
Change control is a complex, multi-step process that requires continuous collaboration between various stakeholders. Each year, biopharma companies initiate hundreds of changes to approved products, many of which have a regulatory impact. Regulatory teams assess those impacts through a separate variation management process and then must work with health authorities around the world to approve the subsequent changes. Currently, quality and regulatory teams manage these processes through disconnected emails and phone calls but that can compromise transparency and lead to critical errors, issues with compliance,
and downstream distribution delays.
The Solution
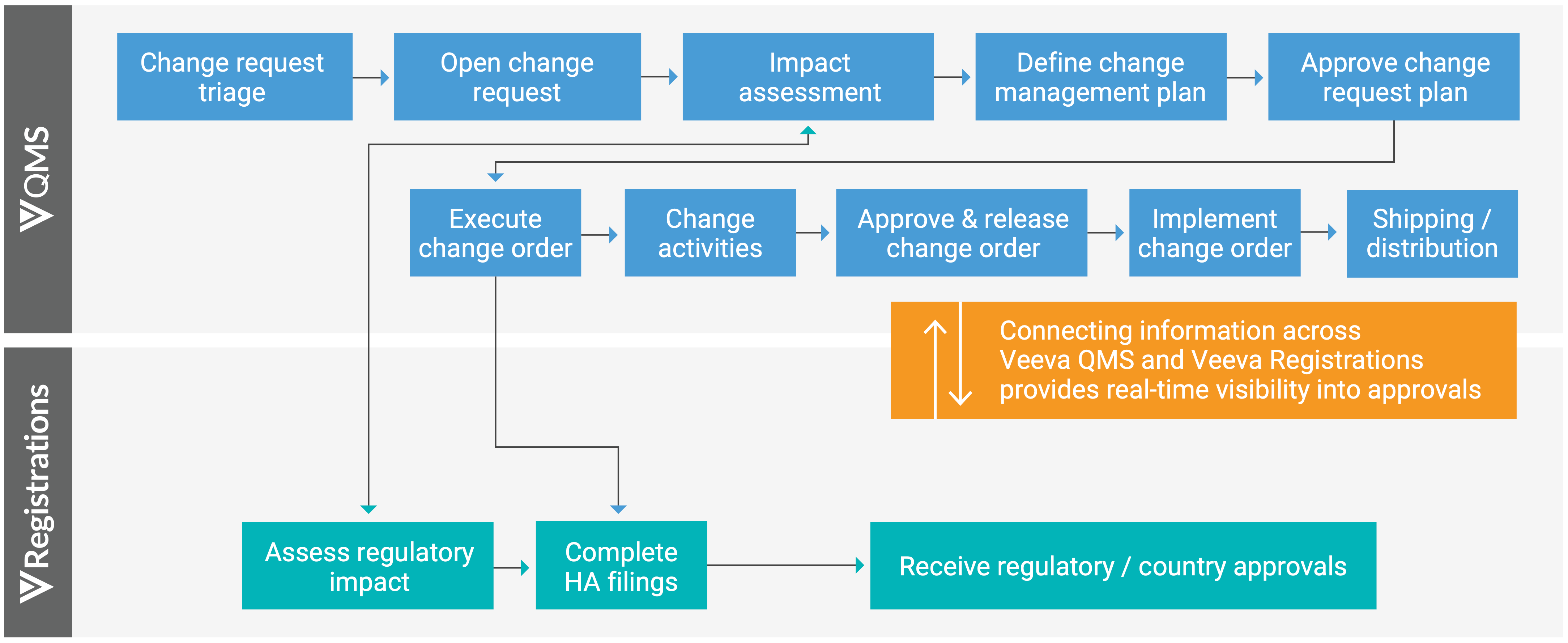
Veeva developed the Veeva Quality to RIM Connection to streamline these two processes. This provides users with real-time visibility into health authority approvals and speeds the change control and variation management processes through a bidirectional process flow.
- A user creates a change request in Veeva QMS and sets off a series of impact assessments on the quality side and license impacts on the regulatory side.
- If the change control is authorized, it moves into execution and that triggers affiliates to complete health authority filings and track their status in the system
- As health authority approvals are received, that information is visible in real-time to the quality team members so they can make accurate product shipping decisions.
Benefits
The Veeva Quality to RIM Connection represents Veeva’s continued investment in innovative solutions to accelerate the quality and regulatory processes. Now, teams can operate with complete impact intelligence, which improves decision making and shortens the overall timeline from change control event creation to implementation. With enhanced visibility, teams are also able to minimize regulatory risks, decrease inventory management issues, and avoid product distribution delays.
