Today, clinical trials depend on increasing
volumes of patient data from more diverse
sources than ever before.
An EDC system produces only about 30% of this data.
To aggregate and clean the 70% of data that streams
in from third-party sources, most data management
teams use manual methods that involve the EDC,
statistical computing environments, email, and
numerous spreadsheets.
These manual methods increase effort, costs, and
potential risk. Taking data offline for cleaning delays
its availability for periods that range from a few days
to a few months, preventing more agile responses
if safety or quality issues arise.
Faster, automated approaches are needed to speed
the availability of clean, consistent clinical data.
An emerging category of solutions, clinical data
workbenches (also referred to as clinical data platforms,
hubs, or data aggregation and management systems),
addresses these challenges.
What are clinical data workbenches?
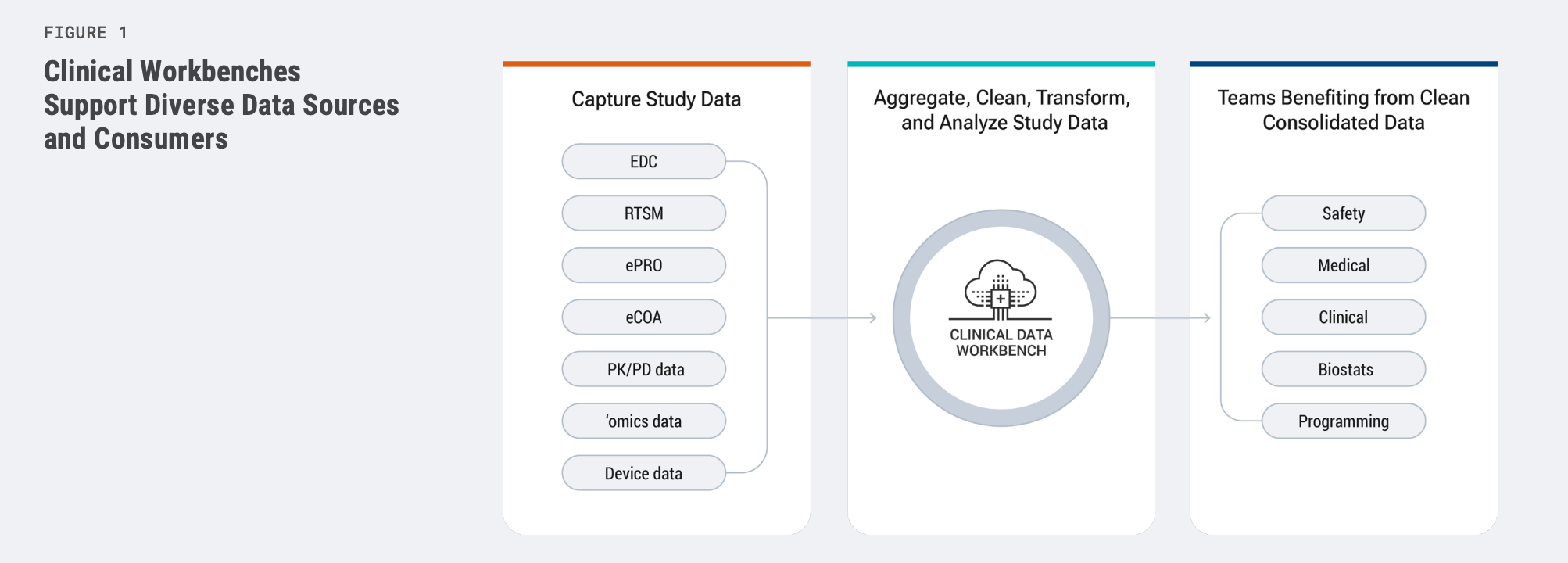
Clinical data workbenches provide a single source of truth for all forms
of clinical data, making it faster and easier for cross-functional teams to
manage the diverse patient data in today’s clinical trials. Workbenches
address challenges posed by the growing volumes of data from external
sources like labs, wearable devices, and ePRO solutions.
They centralize and harmonize the data, and use automation to reduce
reliance on manual processes for transformation and cleaning.
Workbenches also enable better use of analytics, ensure data integrity
and quality, and improve collaboration among different stakeholder
groups, thus facilitating increased trial agility and speed-to-market.
Providing a single, central location for all trial data [Figure 1] improves
accessibility for adjacent and downstream processes such as safety
surveillance or medical and clinical reviews. It also establishes a unified,
harmonized foundation for clinical data to ensure the successful
application of AI and other emerging technologies.
While the first workbench appeared on the market years ago, the
first-generation systems were never widely adopted. Today, there are
new offerings from a diverse range of providers, including Edetek,
Medidata, Oracle, Saama, Signant Health, and Veeva. Each employs
a different model and approach. Across the category, workbenches
serve a wide range of functions, most of which deliver some but not all of
the following: aggregation, review, query management, transformation,
storage, visualization, and artificial intelligence.
The technological advances in this latest generation of clinical data
workbenches deliver a better return on investment than their predecessors.
Sponsors and CROs report seeing improvements from modern
workbenches, including fewer manual processes, automated change
detection, external patient data verification, and reduced cycle times for
query management and database lock.
This guide offers a broad overview of data workbench technology,
summarizing fundamentals for understanding basic system requirements,
developing a business case for investment, and selecting the workbench
best suited to your business needs.
Determining what you need from
a workbench
The first step in evaluating clinical data workbenches is deciding
what your organization needs and how it could benefit most from
this application. The checklist below summarizes the basic business
requirements to consider.
If the data workbench will be a net new system, your data review and
cleaning processes should change to leverage its capabilities. Thus,
when defining requirements, “ask for a car, not a faster horse.” Instead
of seeking to improve a legacy process, strive for greater advances by
investigating what’s possible with these new systems. But verify vendor
claims to ensure your “faster horse” is not a “flying car.”
CHECKLIST
System Requirements
This checklist groups prospective capabilities into the following business requirements:
Data Aggregation, Data Cleaning, Data Transformation, Data Analysis, and System Infrastructure.
Data Aggregation
- DATA INGESTION
Your data workbench should accept trial data from every source used in your trials and require little to no
effort on your part. Be sure that it will ingest data at the frequency you need. Productized integrations between the workbench and preferred data providers add extra value because the providers themselves support them. - EXCEPTION HANDLING
Upon data ingestion, the system should automatically detect issues within the data structure and in the
data file itself. Ask the vendor what types of issues and errors can be automatically identified. Warnings
should be made available to your data management team and vendor. - DATA MAPPING
Ask vendors to describe their data mapping strategies. Utilizing a simple data model will reduce the
number of data mappings that are required. Fewer mappings mean that fewer things that can go wrong
and delay the start of data cleaning and review. - DATA STANDARDIZATION
Standardization generally simplifies downstream activities, but the required data transformations come
at a cost. Strike a balance between easing downstream activities and making data available quickly for
cleaning and review.
Data Cleaning
- LISTING CREATION
Most users want to view data in a tabular form (i.e., as listings), and a workbench should produce tabular
layouts quickly and easily. No-code methods are user-friendly and empower data managers, although sometimes
complexity cannot be avoided. Thus, look for both code and no-code capabilities to create listings. - AUTOMATED CHECKS
Automated checks save time and speed up the cleaning process by automatically identifying data discrepancies
and raising queries about them each time new data is loaded. Look for code and no-code options for writing the scripts. Automated checks contribute significantly to a workbench’s ROI, so the greater the capability, the better. - QUERY MANAGEMENT
Workbenches centralize query management. You should be able to manage and action queries across multiple
data sources. The ability to track existing queries when data is refreshed will prevent users from wasting time updating spreadsheets or re-reviewing data unnecessarily. - QUERY ACCESS
When centralizing query management, you need mechanisms to support communications between systems
and companies. Look for the ability to push and pull queries from external systems, including the EDC.
External data providers should be able to log in to the workbench to view and resolve queries about their data.
Data Transformation
- SDTM OR SDTM-LIKE OPTIONS
While we don’t recommend performing data cleaning on SDTM datasets, the cleaned data must be transformed
into a standardized structure. Consider whether you want to create SDTM or SDTM-like domains within your
clinical data workbench. - DATA EXPORTS, BOTH AD HOC AND SCHEDULED
A workbench should be a conduit through which study data flows. Robust scheduling capabilities allow it to
become part of an integrated process flow, so downstream users can have data exported automatically as often
as needed. It should offer both push (for ad hoc and scheduled exports) and pull mechanisms (via an API).
Data Analytics
- OPERATIONAL DASHBOARDS
Dashboards provide visibility into the completeness and timeliness of cleaning activities. Their tracking and
metrics enable leaders to actively manage the process, improve cycle times, and deliver better service to
downstream data consumers. - DATA VISUALIZATION
Visualizations make it easier to identify anomalies and trends. As with anomaly detection, you may wish
to layer visualization capabilities on top of your aggregated and cleaned data. This capability may exist within
your workbench or via data export capabilities that push the combined data to a dedicated visualization system. - AI/ML ENABLED DATA REVIEW
Artificial Intelligence and Machine Learning capabilities can help identify data anomalies and atypical values at scale. Surfacing anomalous values helps reviewers focus their efforts. Reviewers should be able to drill down from auto-generated lists to the underlying data for further investigation and to initiate queries as needed. - CONSOLIDATED EXPORTS TO AI/ML SYSTEMS
Organizations using AI/ML for clinical analyses can benefit from the clean, consolidated data produced by data
workbenches. Ensuring that ML models for RBQM are trained against clean data improves the output quality.
Ensure your workbench provides granular controls and scheduling capabilities for data exports. - PATIENT PROFILES
Your workbench should provide a comprehensive and up-to-date status of data cleanliness for all data collected at the individual patient level. A patient-centric view allows data managers to prioritize time and resources and lock patients faster for interim analyses, giving medical and safety teams visibility and perspective for better decisions.
System Infrastructure
- SCALABILITY
When evaluating scalability, estimate the number of studies you will run over the next five to seven years and how
much data they will contain. The workbench must be able to handle the highest volume of data expected from your
largest studies, as these are often the most important, and have the most to gain from a workbench application. - PERFORMANCE TESTING
Any workbench may perform well when demonstrated on a single study that involves small volumes of data, but
how will it hold up in a mega trial? Ask vendors about their performance testing and for references from customers
who’ve used the technology to handle similar volumes of data. - SYSTEM INTEGRATIONS
Workbenches sit between data providers and data consumers; therefore, an open, well-documented API is
imperative, and productized integrations are highly valuable. Engage your IT team to evaluate how easy or difficult it will be to integrate this workbench with other systems. - SERVICES AND TRAINING
Are you buying an off-the-shelf product or a services-based solution? Once the workbench has been implemented, will you need ongoing vendor services? If so, why? What level of service will be required for each study? How much training would your teams need to become self-sufficient in configuring the system for each new study? - SLAs AND SUPPORT
Ask about the Service Level Agreements (SLAs) related to system up-time and help desk responses. If your studies
never sleep, your workbench system and its support team shouldn’t either. - DATA SECURITY AND COMPLIANCE NEEDS
Having aggregated all of your study data in one place, you need to be confident that it is secure. Make sure any
prospective workbench vendor passes a rigorous security audit.- Do they have systematic approaches to security architecture and follow best security practices from an
organizational and personnel perspective? - Do they have clear network communications and encryption practices and perform regular vulnerability and
penetration testing? - Have they established procedural information privacy controls?
- Are they open and clear about how they’ll look after your data?
- Do they have systematic approaches to security architecture and follow best security practices from an
Maintain a healthy dose of skepticism
when evaluating options
Some solutions in this space rely heavily on services-developed
customizations to deliver functionality. Customizations can meet
highly specific requirements and provide cutting-edge capabilities,
but they also result in greater fragility. That fragility increases the
ongoing maintenance costs for new trials and software upgrades.
Approach workbench evaluation with a healthy dose of skepticism
and request external validation and proof of any messaging claims.
Place extra value on referrals and testimonials from current satisfied
customers, along with the ROI they have achieved.
Ensure that vendors are transparent about the following:
- What their products can and cannot do today, out of the box.
- What can be done with customization.
- What they plan for future releases.
- What features have been delivered over the past 12-18 months.
Since services teams can add virtually any capability, services-heavy
offerings can deliver false positives to RFP questions asking:
“Can your system do…?” Ask vendors to specify which capabilities
are part of the “out-of-the- box” offering and which are achieved
through customization.
What is the pricing, and what are the
commercial terms?
As you’d do when comparing electric and gasoline-powered vehicles,
evaluate the Total Cost of Ownership (TCO) rather than just upfront
licensing costs. A workbench’s TCO should include licensing,
implementation and training costs, projected system integration
costs, maintenance fees, renewal costs, and all likely service costs
over five years.
Making a business case
Gaining approval for a new business system takes work. To increase the
likelihood of approval, articulate existing challenges and their impact,
along with the anticipated value of your proposed solution.
Focus your business case on big-ticket items for the key decision
makers: for programming, it’s the time spent to generate listings; for data
management, it’s the manual validation checks and query management;
and for safety and medical teams, it is the impact of delayed data.
A good business case should include the following five items:
- PROBLEM STATEMENT
Define the specific business problems and pain points the new
system will address. List the challenges and delays inherent in
manual processes and bring them to life with quantification and
anecdotal examples. Show the limitations and impact of the
existing processes. - PROPOSED SOLUTION
Outline the proposed solution and its key features. Explain how
these features will address the identified problems and deliver
tangible benefits to the organization. Describe the system’s core
capabilities and alignment with the organization’s strategic goals. - QUANTIFIABLE BENEFITS
Quantify the expected benefits of the new solution in terms
of cost and time savings, productivity improvements, and
quality-of-service measures. For example, how quickly can
medical and safety teams access reliable data? Use
quantifiable gains, such as fewer hours spent on manual
validation checks, to demonstrate the system’s potential
return on investment (ROI). - IMPLEMENTATION REQUIREMENTS
Define the key steps to deploy the system, train users, and
deliver the change management needed for a smooth transition.
At this point, a detailed implementation plan isn’t realistic.
For the business case, define a realistic plan that provides a
reasonable estimate of the resources required. Get input from
IT on past costs based on the expected effort required. - SUSTAINABILITY PLAN
Describe the ongoing support and maintenance needed for the
new system. Outline the costs associated with ongoing support,
upgrades, and security measures. If incremental configuration
or service costs are necessary for each new study, ensure
those are captured and included. A realistic sustainability plan
contributes to long-term system viability and a credible business
case for making the investment.
Take action
Clinical data workbenches are gaining the attention of clinical data
management leaders. They provide an attractive solution to challenges
that have existed for many years and that are only getting worse. If you
don’t already have a workbench, now is the time to start planning for one.
Clinical data workbenches offer an alternative to legacy SAS-based
manual aggregation, transformation, and cleaning to help ensure
clinical data quality, regulatory compliance, and patient safety. The
latest generation of workbenches is already demonstrating ROI by
saving time, improving efficiency, and enriching collaboration between
clinical trial stakeholders.
Delivering value today and tomorrow
As trial designs become more complex and the volume and variety
of sources for patient data increase, workbenches are emerging
with the data science tools needed to clean, transform, and analyze
clinical data faster and with less effort. Study teams gain quicker
access to a stream of clean and consistent data, helping them be
agile and make better decisions, contributing to patient safety
and trial efficiency.
In addition to providing immediate efficiency gains, a workbench
provides a foundation for adopting new technologies that support
adjacent business processes. Today, it is difficult to layer AI/ML and
other emerging technologies on a siloed, manual infrastructure for
clinical data. A modern foundation for clinical data readies the
organization for future advances in data management and beyond.
This ebook provides more insights into today’s evolving clinical data landscape.
