4 Ways Top Sponsors
Build Strong and Sustainable
Site Relationships
Download PDF
Over the past five years, there’s been an explosion of new technology introduced to improve clinical research site engagement. But these well intentioned solutions are often custom, standalone systems that require complex middleware and integrations. This forces sites to adopt dozens of sponsor-specific tools and build customized processes around them. This is just one of the reasons an estimated 3,000 clinical research sites have stopped conducting trials since 2019, according to Ken Getz, executive director of the Tufts Center for the Study of Drug Development (CSDD).
Although most sponsors understand the urgency and value of improving site relationships, it can be challenging to implement effective strategies that don’t exacerbate existing technology siloes. In this report, clinical operations and site engagement leaders at top 20 pharma companies share how they’ve improved site collaboration despite these industry challenges.
Unpacking current conditions for clinical research sites
The majority of the 3,000 sites that stopped conducting trials were small, community-based independent centers running one or two clinical trials annually. “This exodus of lower volume and less experienced sites with modest infrastructure has caused larger sites, health systems, and academic medical centers to pick up more activity,” says Getz.
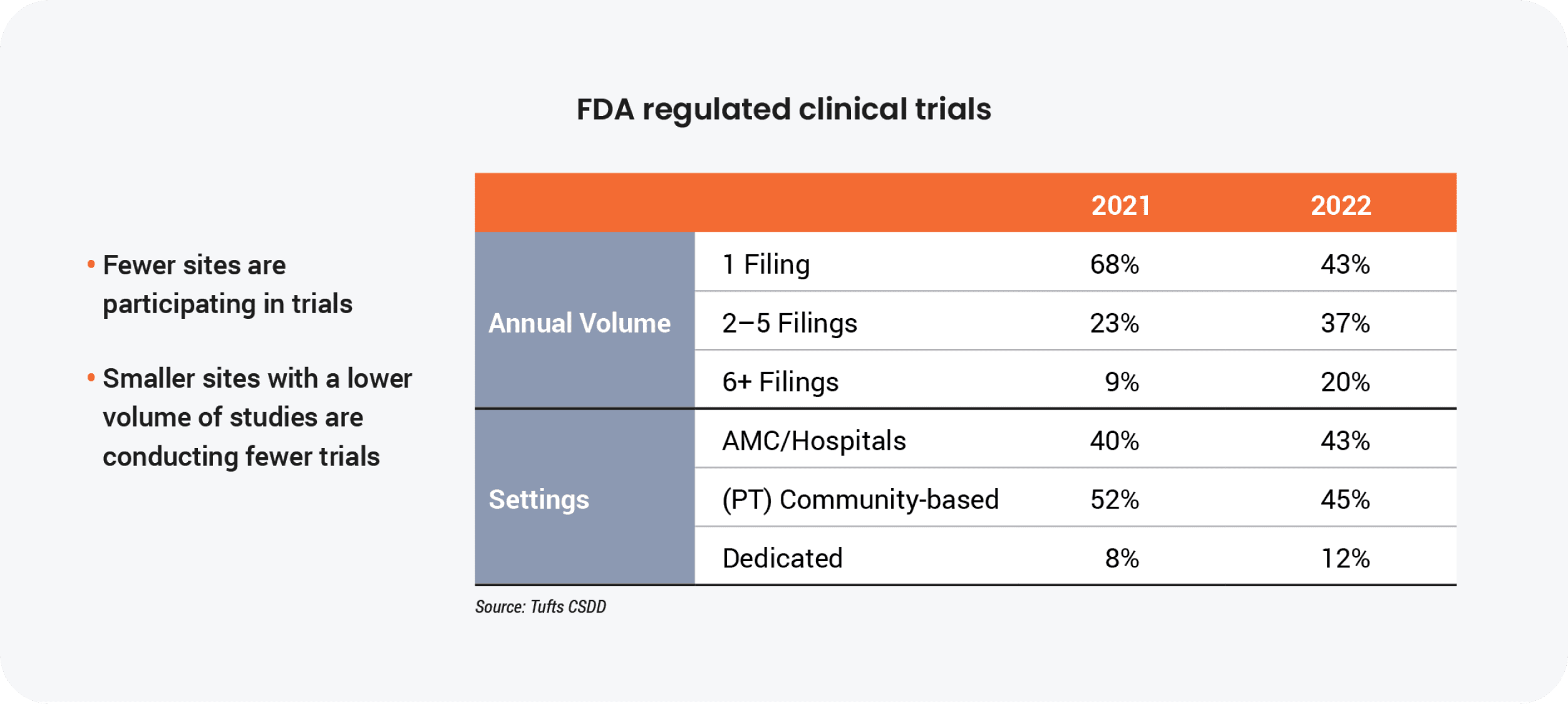
Protocol complexity is a main contributor to site consolidation. The sheer volume of data and the proliferation of technology over the last decade have made it difficult for smaller sites to keep pace with industry trends.
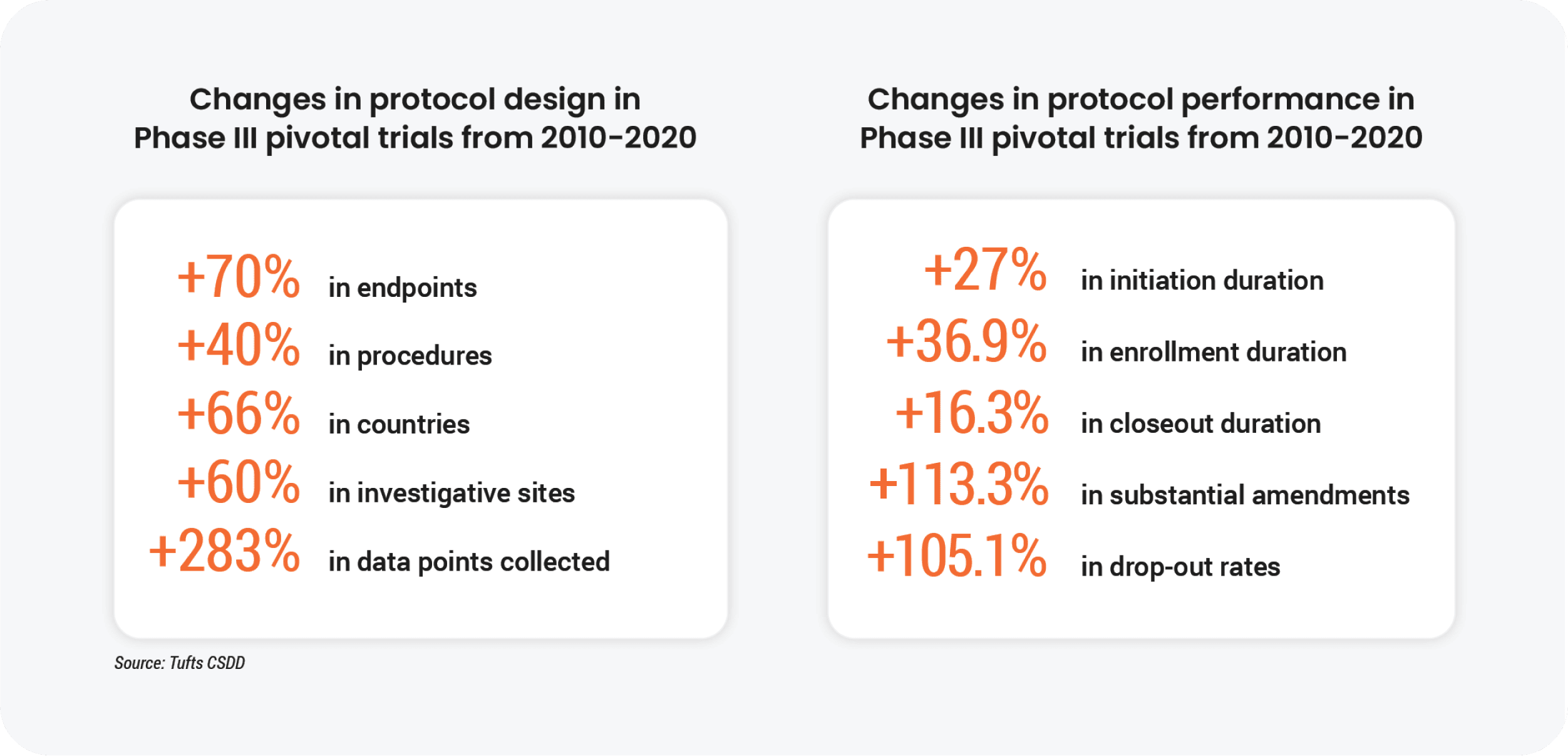
These complex protocols worsen existing technology silos for sites. According to a Society for Clinical Research Sites survey, over 60% of sites use more than 20 systems daily. On average, site staff spend 5-15 hours per month learning how to use new technology. For busy research coordinators, this technology burden detracts from their top priorities: patient scheduling and being present during visits. Over-reliance on email and repetitive communication with sponsors and CROs limits the time site staff can spend with patients, often delaying start-up of vital studies.
Despite protocol complexities and dwindling site numbers, global sites are optimistic about clinical trials. A 2023 Tufts CSDD survey found that site optimism is significantly related to the degree to which site staff feel that sponsors value and apply feedback from sites. This highlights the crux of the issue: an engaged site is one which has an open and collaborative relationship with its sponsors. Here, several leading sponsors demonstrate four key methods for improving site engagement:
An engaged and thriving site is one which has an open and collaborative relationship with its sponsors.

#1 Standardizing the site experience
Creating a consistent user experience is a top priority for most sponsors: they need a standard method for engaging with sites that allows flexibility toward the site’s preferences.
The SSO strategy and operation manager at a top 20 biopharma wants to create an intuitive experience for sites. She explains how this approach would benefit them: “Sites need an easy system that they can navigate independently. They should be able to bulk upload documents and find the information required to support patients in real-time during the trial.”
The need for standardization is compounded by the number of different systems and passwords that sites must navigate daily. “We regularly hear from sites that each tool that sponsors provide requires knowledge and training,” says the head of clinical trial management services and solutions at one top 20 biopharma. “Being able to provide a standard across sponsors removes some of the burden from sites.”
Another top 20 biopharma has a similar goal: become less sponsor-centric and give sites more choice when it comes to technology. The company’s head of clinical software development (COE) comments: “We think we are bringing the right solutions to sites, but it’s not just about our relationship with them. Sites are working with all of us.”
Unified solutions that automate information flow across trial partners, processes, and systems are improving site collaboration without adding to the technology burden. These tools should have features like:
- Single sign-on to give sites easy access to all sponsor technologies through one ID
- A simple, free app to easily manage site content like ISF and delegation logs
- Streamlined information and data exchange to improve collaboration between sponsors and CROs
- High-value training where sponsors assign tasks automatically before site visits and track on-site activities

#2 Building collaborative relationships with clinical research sites
As an industry pioneer in site-centricity, Merck & Co., Inc., Rahway, NJ, USA (hereinafter “MSD”) has worked to address the perception that sponsors have a top-down approach to their relationship with sites and do not consider their needs. Natalie Blake, senior director of MSD’s project management office at the global clinical trials organization (GCTO), explains the company’s approach: “To effectively partner with sites, we must give them a seat at the table and incorporate their valuable input into MSD’s decisions as a sponsor.”
MSD is moving away from process-driven site-sponsor relationships toward a consultative and dynamic mindset. To support this shift, the company implemented the Clinical Site Partnership (CSP) program, leveraging a network of 30+ global sites to develop clinical trial technology strategies that accommodate all study partners. CSP includes focus groups, live site observations, surveys, workshops, and user experience sessions.
Since implementing this program, MSD has garnered feedback on over a dozen initiatives in areas like clinical supplies and data management. The company also implemented a “menu” of operational enhancements to make it easier for sites to conduct clinical trials. CSP sites perform well with patient enrollment and comprise over 20% of MSD’s oncology portfolio.
Sites participating in the initiative say that CSP opened lines of communication between MSD and monitors. Dr. Mustafa Erman, head of preventative oncology at the Hacattepe University Cancer Institute, describes his experience: “Being a part of CSP has allowed me to communicate freely and frequently with MSD to optimize processes. It’s also improved screening, recruitment, and patient care.”
Clinical research sites in MSD’s partnership program enroll over 20% of the total oncology portfolio.

#3 Investing in site-centric technology
Part of building collaborative relationships with sites is providing technology that empowers success at every stage of the clinical trial lifecycle. A top 20 biopharma has dedicated resources to improving site collaboration and accelerating study start-up processes. Two recent initiatives have focused on improving study start-up cycle times.
In July 2023, the clinical operations team launched a program-specific campaign using Veeva Site Connect to automate document exchange with eight studies and 1,600 sites. Since then, they’ve seen:

To ease the process for sites, the biopharma designated sponsor points of contact to provide direct site support. They also created a centralized location for all system processes and documents, including an FAQ document and a question-and-answer service.
All sponsors strive to get sites up and running as quickly as possible, but how can they without data that actually measures the company’s start-up cycle time? The top 20 biopharma launched its second initiative: a joint effort between study start-up leaders and executive leadership to select KPIs that would provide better visibility and actionable insights to reduce cycle times. They identified metrics across 175 studies and created dashboards for each KPI to monitor progress by therapeutic area, country, and study. Using Veeva Site Connect and Veeva Study Startup together, the team was able to decrease site selected to site activated cycle time by 30% and decrease the time from when the start-up package is sent to activation by 36%.
The company continues to measure data to identify and implement process improvements, and make data-driven technology decisions.

#4 Better site training unlocks trial efficiency
To engage sites and streamline processes during study start-up and conduct, sponsors are looking toward optimizing site training. The global clinical capability strategy lead at one top 20 biopharma advocates for site simplicity and is focused on providing a user-friendly study training experience that meets the needs of individual sites and studies.
“I’m very mindful of the fact that site staff need to spend most of their time with patients,” she says. “As sponsors, we should ask ourselves: ‘How can we be more targeted in the training we deliver and our expected outcomes?’”
The strategy lead sought an automated, end-to-end training process in a validated system with these objectives in mind. The biopharma became an early adopter of Veeva Study Training, which automates and streamlines training for sponsors, CROs, and sites in one system. Their team implemented a multi-method approach to study training, offering pre-requisite reading of key study documentation, recorded modules, as well as targeted virtual and face-to-face interactions where there is additional study complexity.
The team has seen an almost 100% adoption rate among site users and provides the following tips to engage sites.

Sponsors who choose not to invest in technology can still prioritize site-centricity by establishing an advisory board or leveraging positive site relationships to gather input on trial decisions.
A connected environment for all
Making a strategic effort to refocus on the fundamentals is the key to improving site collaboration in the long term. Engaging sites and giving them a seat at the table will lessen the transactional nature of site-sponsor relationships and streamline study execution. The site-centric approach can be straightforward: prioritize site input and foster collaboration to ensure better clinical trial outcomes for all.
Veeva Clinical Operations provides an end-to-end environment and collaborative platform for sponsors, CROs, and sites. With integrated and automated workflows for start-up, training, execution, and close-out, clinical trials are accelerated with more efficient communication among cross-functional teams.
“That’s why we believe in Veeva,” concludes the top 20 biopharma’s head of clinical software development. “To get an industry standard for sites.”
