Blog
Implementing Study Amendments Without EDC Data Migrations
Oct 25, 2019 | Richard Young
Oct 25, 2019 | Richard Young
Revolutionizing database design with modern EDC
The Challenge in Clinical Trials Around Migrations:
The Tufts findings on clinical trial complexity demonstrated how the rising number of protocol amendments hampers our ability to deliver clinical trials. They concluded that a Phase III study had an average of 3.6 protocol amendments, each containing 8.5 changes. That is over 30 individual changes in total.
This number of database changes does not include other changes, such as corrections, updates to procedures, addition of edit checks, etc. If we were to consider the total number of structural and functional changes required, the count would surely be much higher.
Applied Clinical Trials published an article on how the complexity of the study and CRF designs directly correlates with the number of changes needed in the eCRF. For the most complex studies, on average 17 study migrations were required. Why are “migrations” a big challenge that we need to be especially aware of?
In 2015, Joris De Bondt, SGS, published an approach to amendments that concluded that a simple change typically took 10 days to implement and a complex set of changes would typically take much longer.
“Mid-study updates can have limited to wide impact on the eCRF. The typical turn-around time for implementing a limited change, for example, adding 1 existing form in a new visit (folder), is 10 working days.”
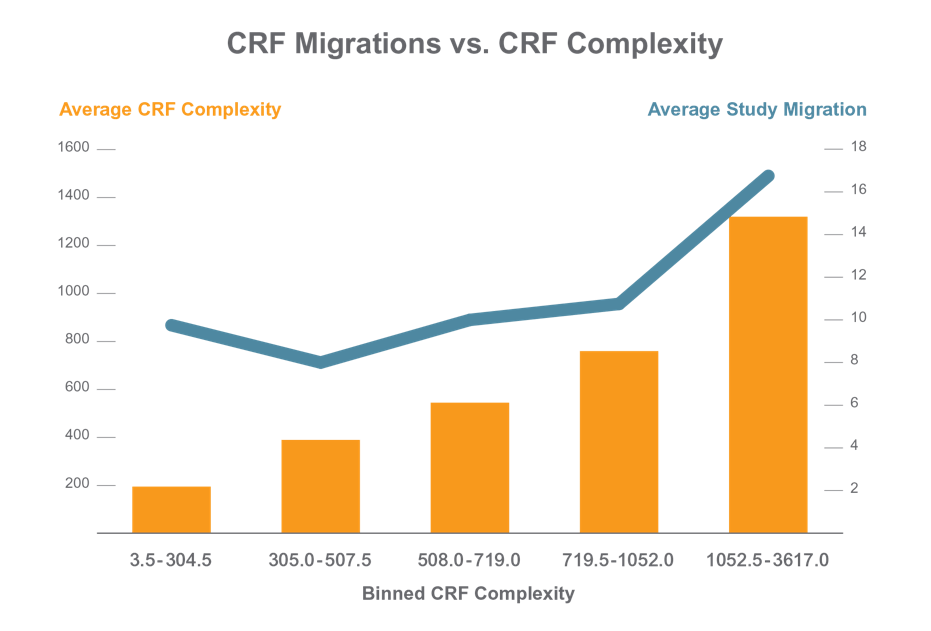
Reference: http://www.appliedclinicaltrialsonline.com/time-another-crf-migration
The key challenge is that in order to make these changes, traditional EDC systems need the organization to build a new database and physically move the clinical data. To achieve an effective data migration procedure, data on the old system is mapped to the new system. Programmatic data migration may involve many phases but it minimally includes data extraction from the old system and data loading to the new system.
After loading into the new system, results are subjected to data verification to determine whether data was accurately translated, is complete, and supports processes in the new system. During verification, you may need to run both systems in parallel to identify disparities and forestall erroneous data loss.
During this process, it is necessary to halt work in the original database, while these time consuming processes are effected. In other words, downtime abounds. Each migration requires study downtime ranging from several hours to several days, causing delays in the study.
In their 2014 article, one of the traditional EDC providers advised sponsors and CROs to simplify their eCRFs in order to minimize migration costs and downtime. However, dumbing-down eCRFs creates risks around data quality, sub-optimal study designs, and poor alignment to increasingly complex protocols.
This situation underlies the need for a better EDC, one that not only simplifies change management but embraces it. In an era of adaptive trials and precision medicine, we will be making changes more frequently than ever and must be able to implement them in real-time.
A Modern Architecture and Approach
A modern EDC allows for a casebook design that is as dynamic as the clinical trial itself. Veeva has delivered a next-generation EDC system, designed specifically to address the migration issue. Data is stored in a manner that provides flexibility and removes the need for migrations entirely. The ability to insert new data collection elements, new pages, and new visits, without bringing the system down or migrating data, simplifies the initial build as well as subsequent amendments.
A Flexible Architecture that Supports Change
Taking this further, not only does the build process create EDC, but also creates the site source documents – eSource. This results in one build for both EDC and eSource with one amendment process.
With Veeva, all visits, forms, and fields are defined using a single data model with database tables and columns that do not change between studies. New studies and their eCRFs are defined by adding rows to the existing tables and columns. Once the study is live, we can add or change elements by adding new rows, without changing the database structure.
EDC uses reference IDs as keys to the underlying data rather than use the physical location of the data. The clinical data that gets entered is stored with a reference to the definition data for that field. When a field is changed, a new reference is created for the stored value. As a result, the system can have multiple versions of a casebook at any time, there are no changes to the database structure, and study migrations are obviated.
The Vault architecture supports multiple CRF designs while adding/updating only the net changes. Any element of instantiated CRFs that are unchanged in design are completely untouched when deploying an amendment. This ensures that only objects with modified designs are affected. Additionally, updates to implemented CRFs only require a simple reference change to the new definition. Every casebook can reference a different definition if need be, so this dynamicity extends to the subject level rather than just the study level. This design enables you to update individual sites and even individual subject casebooks with no site-level downtime.
Deploying Changes Without Disruption
Vault EDC saves you time and money by implementing changes in near real-time. When deploying a change, we can automatically lock users out of a casebook for the few minutes it takes to effect the change and then release the page. Normal service is resumed within minutes, not hours or days later.
Disruption to sites is minimal if at all. Vault EDC allows scheduling changes in advance so they are implemented during non-working hours at each site. Prior to making a change, running an impact analysis report will help you review changes and educate site personnel effectively on new and updated requirements.
The benefits from this flexibility are considerable: the desired changes go into effect faster, administration costs are minimal, and downtime for sites is eliminated. Perhaps most importantly, making amendments no longer poses risk to your data.
See how with a modern, cloud-based architecture, Veeva Vault CDMS enables life sciences companies to amend the clinical study database with total ease and zero downtime. Watch a 5-minute demo here.