Blog
Tips for Managing the Brexit Transition Within Your Organization
Nov 24, 2020 | Renee Menco
Nov 24, 2020 | Renee Menco
The United Kingdom formally left the European Union on January 31, 2020, and we are quickly approaching the end of the Brexit transition period on December 31, 2020. Because Brexit impacts every sponsor that has marketed products in the EU and wants to continue marketing them in the UK, the Veeva team is working with customers to ensure they can manage this transition seamlessly.
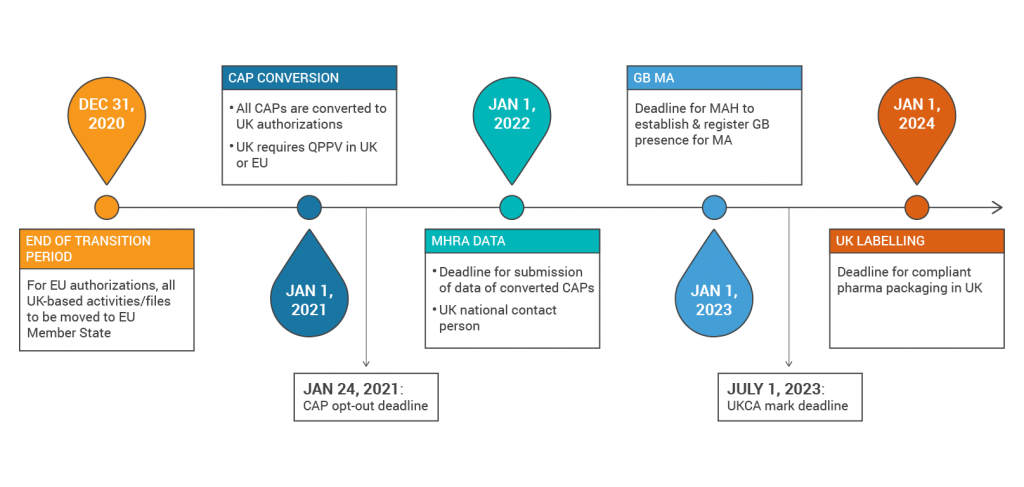
The first major milestone that sponsors need to consider is that MHRA is converting all existing Centrally Authorised Procedures to UK applications on January 1, 2021. Sponsors will then need to submit an initiating sequence (representing the currently authorised product), a list of historical regulatory activity, the SmPC, package labels, and leaflets to the MHRA by January 1, 2022. All products approved in the UK via MRP/DCP will remain valid and receive a UK MA number on January 1, 2021, but sponsors need to remove the UK from the MRP/DCP prior to that time.
In addition, for all EU procedures, the Marketing Authorisation Holder (MAH), Qualified Person for Pharmacovigilance (QPPV) and Pharmacovigilance System Master File (PSMF) locations must reside in the EU. If marketing in the UK, the MAH must be located in the UK by January 1, 2023. The QPPV and PSMF location may be in the UK or EU, but if the QPPV is in the EU then the MAH must nominate a national contact person.
Organizations that manufacture medical devices should also be aware that the CE mark will only be recognized by MHRA until June 30, 2023. After that time, the UKCA mark will be required. Starting on January 1, 2021, all medical devices and IVDs placed on the UK market will need to be registered with the MHRA, although there will be a grace period for registering depending on the classification of the device. Foreign device manufacturers will need to establish a UK Responsible Person by January 1, 2021 who will be responsible for acting on behalf of the manufacturer, including registering the device with MHRA.
Below is a summary of the Brexit timelines:
Veeva will continue to monitor changes in this area and work with sponsors to overcome the challenges that evolving regulations can create. We will provide our Veeva Vault RIM customers with guidance and best practices for compliance, and we will empower them with the information they need to take action in a timely manner.
To see how Vault RIM helps sponsors streamline global regulatory processes, watch this six minute product demo.