Fast and Successful EDC Study Builds with Veeva Clinical Data
30 eCRFs, 22 built directly from the protocol
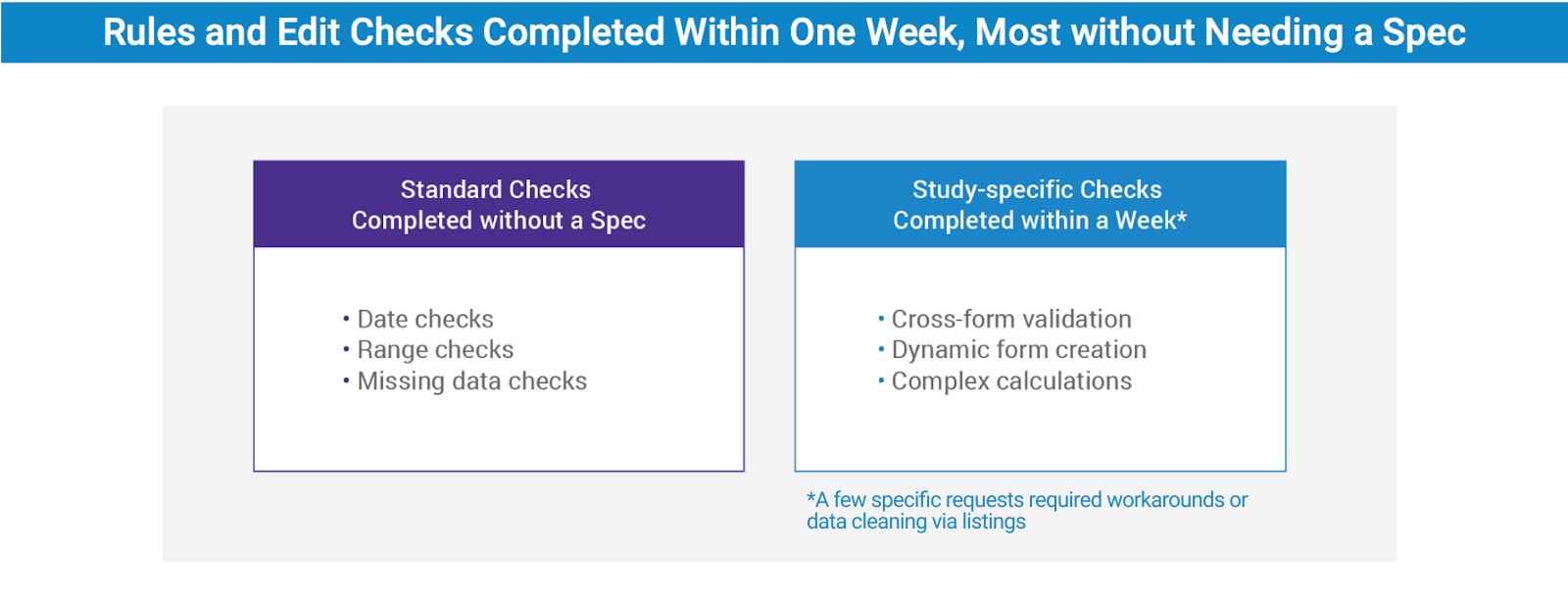
Rules and edit checks completed within one week
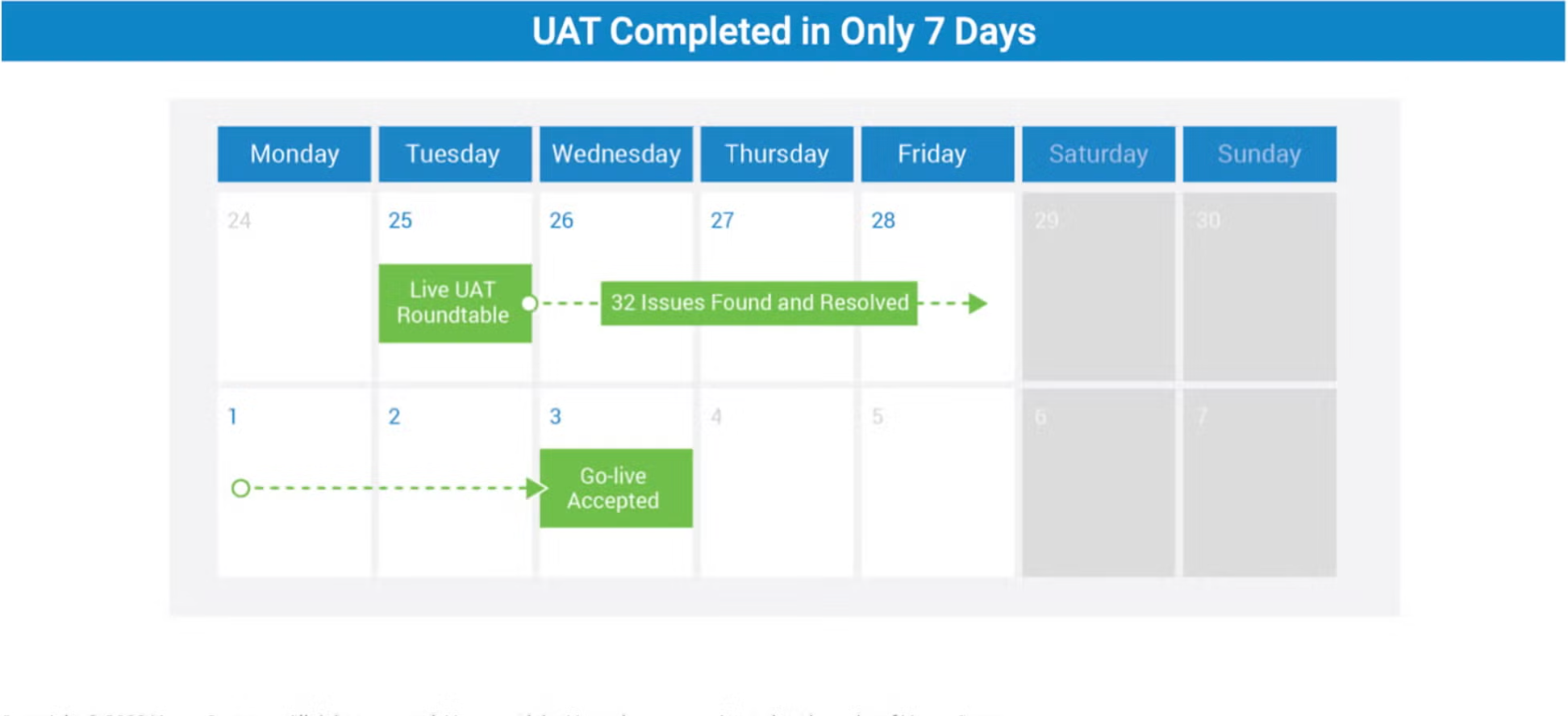
UAT completed in 7 days
Most lay people may think that late-stage change requests are the exception and not the rule, but if you poll data managers you’d hear they are the norm. That was the case for this 400-person biopharma.
During their first study with Veeva EDC there was a late-breaking shift in strategy. Their Phase II early stage feasibility study in a new indication turned into the program-leading first trial. As they were finalizing the database build, the Medical Director recommended that they establish data collection standards for the new indication—starting with this first study.
“It was a total change in mindset and late in the game. It would be a crunch to maintain existing timelines.” – Director of Data Management, Multi-national Biopharma
The People, Processes, and Technology that Made it Possible
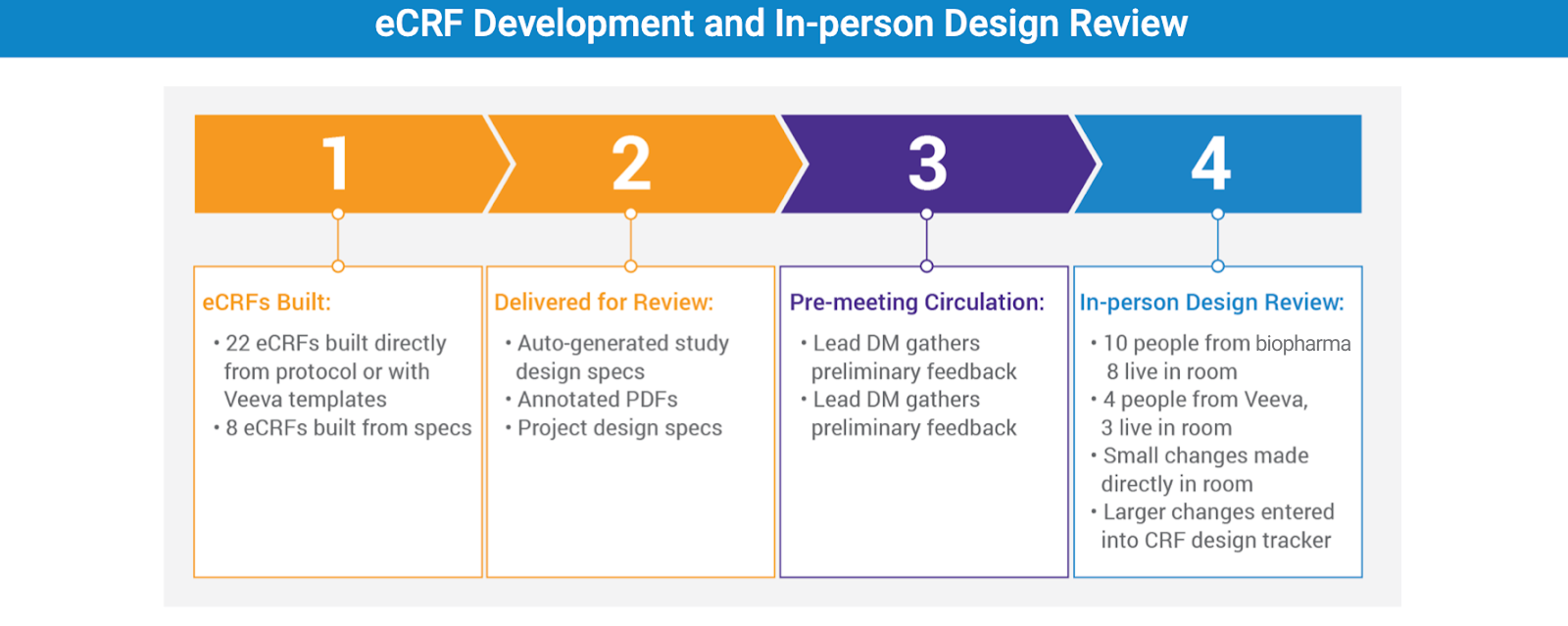
Veeva’s Agile Design process for building case report forms (eCRFs) was particularly helpful. Of the thirty final eCRFs, twenty-two were built directly from the protocol. Standard edit checks (dates, ranges, and missing data) were completed without needing specifications. The study-specific checks, including cross-form validations and complex calculations, were completed within a week.
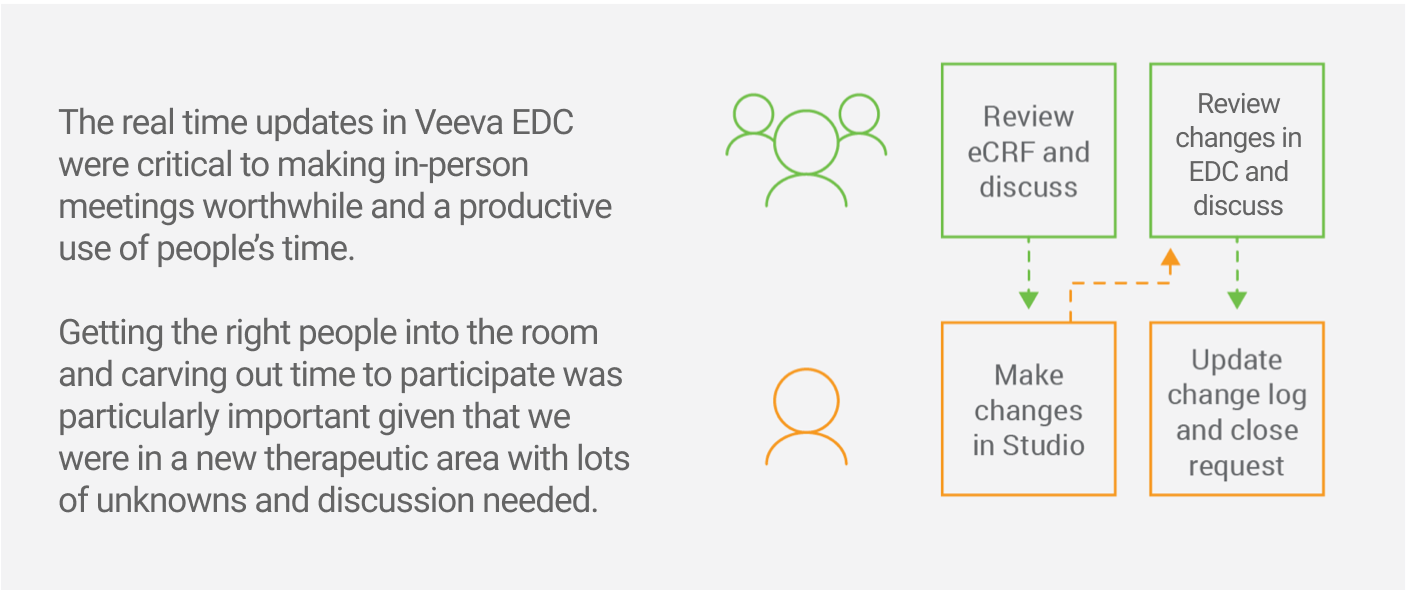
An Interactive Design Review Meeting with Real-time Updates to the Study
“Data collection designs can be sub-optimal at times when programmers work strictly from specifications. We appreciated having study designers who were also data managers building our forms.” – Director of Data Management, Multi-national Biopharma
Once the eCRFs were completed, Veeva sent annotated PDFs, an auto-generated specifications document, and links to the live development environment where people could review the actual casebook forms. The lead data manager gathered preliminary feedback, which Veeva incorporated prior to the live Design Review and Decisions Workshop.
In the workshop, study team members were able to see in real time what their proposed changes looked like. The director of data management recalls, “There was lots of back-and-forth between our internal functions. We’d say, ‘Is this what we want? No, could we make it look like this? Oh, well, on second thought… let’s make it this way’.” Given the new therapeutic area, there were lots of unknowns and discussion needed. This made seeing and discussing the updates in real time critical to hitting their deadline.
“Most of the changes were implemented right in front of our eyes, which was extremely helpful to making decisions and hitting our deadline.” – Director of Data Management, Multi-national Biopharma
Throughout the development process, the teams were in close communication. The sponsor had direct access to the development sandbox, and could review and approve the updates as work progressed. Rather than use email, they kept a shared online change log. The sponsor used the log to specify requests and Veeva used it to request clarifications and track completed work. The shared view allowed both teams to move quickly and keep in lockstep regarding what had been implemented and what hadn’t.
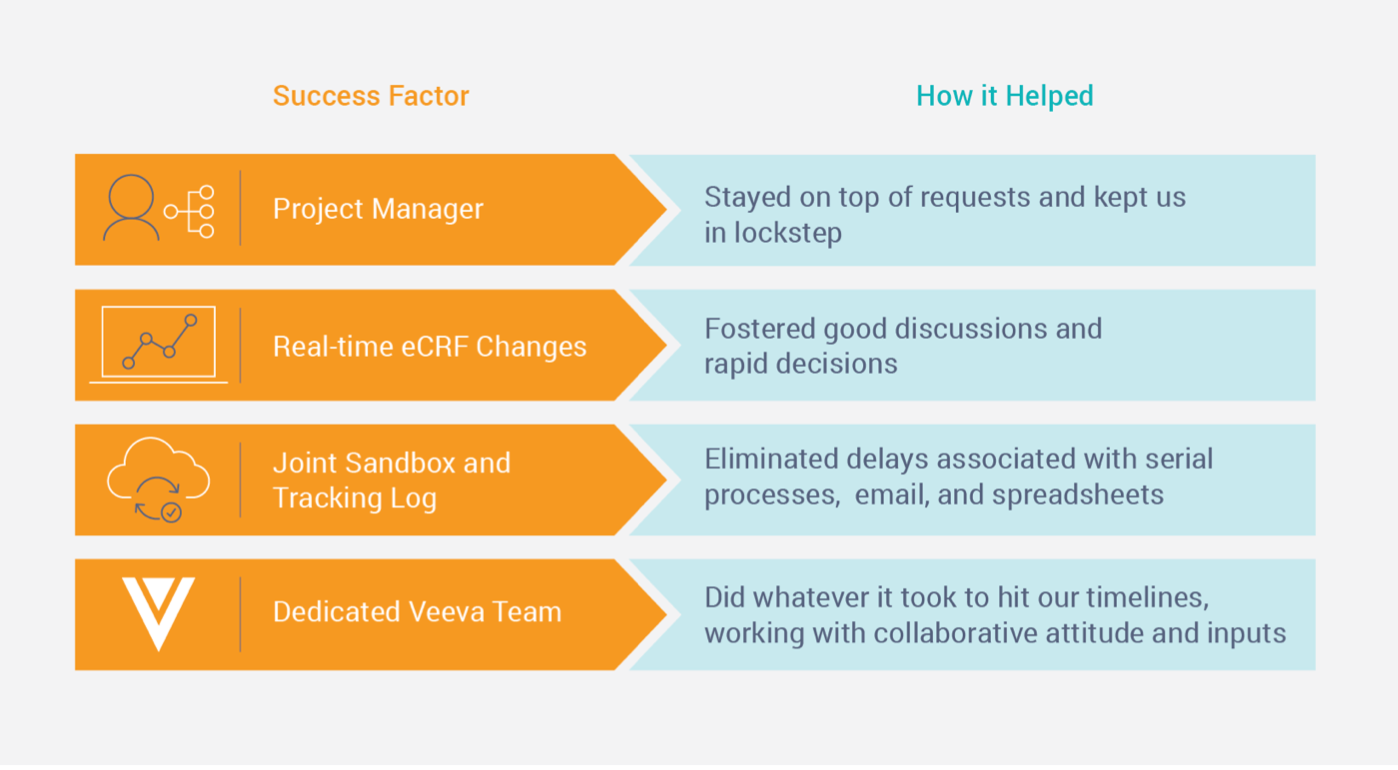
Facing Last-minute Changes – Four Keys to Success
Overview: An Agile Design Process with Veeva Clinical Data