Site Training Soars at Bayer
with Veeva Study Training
42
ongoing studies using Veeva Study Training across 50 countries
1,000+
sites and 10,000+ site users
~100%
site adoption rate for new studies on Veeva Study Training
Biopharma companies face a long-standing industry challenge of training study staff and site personnel in an efficient, cost-effective, and compliant way. Site staffing requirements are some of the most expensive components of a clinical research program, so streamlining training to minimize risk is vital for sponsors’ bottom line.
Ellen Vanderlinden, global clinical capability strategy lead at Bayer, is on a mission to enable both internal study teams and site staff to produce the high-quality training compliance data needed for the company to support the delivery of therapeutic products. “Veeva Study Training really is our foundation to support electronic study site training documentation,” Vanderlinden explains. “We have the end-to-end compliant solution that we were looking for.”
Nowhere to scale
The increasing volume and complexity in clinical studies make it challenging for sponsors to manage training assignments and records manually. Compliance errors are more likely without a connected system that enables study teams to oversee assignment completion and automate training documentation.
For years, Vanderlinden’s team relied on costly external vendors for study-specific training, which focused on face-to-face training in a one-size-fits-all model. Vanderlinden recalls the typical approach: “Training site staff came at a high cost for us as a sponsor. We were transporting doctors from around the world to a central location for them to spend entire days in meeting rooms, presenting slide after slide. This didn’t consider the knowledge, experience, and the specific role of each member of staff in the study.”
Clinical research associates (CRAs) would often have to repeat training for site staff that could not join the live session. They also relied on wet ink paper sign-off of training documentation. “It was very resource intensive for sites and sponsors,” says Vanderlinden. “Ultimately, this approach gave us no oversight nor further understanding of how the training was received and objectives understood.”
Spurred by the COVID-19 pandemic, Vanderlinden’s team brought the electronic training documentation solution in-house and worked to scale up with a non-validated internal system. A benefit of this approach was that learners could complete training online without having to travel offsite. But many manual processes were still required for the study teams to produce compliant documentation: from disconnected Excel trackers and SharePoint files to paper logs to track training. After manual sign-off, it would be filed to their Veeva Vault eTMF.
Enabling sites through targeted training
Vanderlinden is an advocate for site needs and is focused on improving the study- specific training available to them. She had two key objectives when devising a new study training strategy:
- Meet the needs of individual sites and studies
- Implement a simple, efficient, and user-friendly platform
“I’m very mindful of the fact that site staff need to spend most of their time with patients,” Vanderlinden says. “As sponsors, we should ask ourselves: ‘How can we be more targeted in the training we deliver and our expected outcomes?’”
“Veeva Study Training really is our foundation to support electronic study site training documentation.” – Ellen Vanderlinden, Global Clinical Capability Strategy Lead, Bayer
Vanderlinden sought an automated, end-to-end training process in a validated system with these objectives in mind. Bayer became an early adopter of Veeva Study Training, which enables companies to automate and streamline training for sponsors, CROs, and sites in one system. Vanderlinden’s team introduced the system in 2022 and implemented a multi-method approach to study training, offering recorded training modules and pre-requisite reading of key study documentation, as well as targeted virtual and face-to-face interactions where there is additional study complexity.
Bayer currently has 42 ongoing studies using Veeva Study Training across 50 countries. Of those, 11 are using the platform for site staff training, which includes about 10,000 users from 1,000 sites across 37 countries.
“One of the key criteria for us to work with Veeva was that it’s an easy-to-use system,” explains Vanderlinden. “On a practical level, we can leverage our connections with the Veeva Clinical Platform. We use the training material, site staff information, and site readiness status stored within the Veeva Clinical Vault Platform. This was a key opportunity we saw for implementing Veeva Study Training.”
A centralized team is responsible for logistics, set-up, and back-end support, and that same team managed the migration of select records from the existing internal system. “For us, the centralized team worked very well,” says Vanderlinden. She adds: “We wanted to ensure a consistent approach to migration and avoid burdening the study teams.”
“We use the training material, site staff information, and site readiness status that’s stored within the Veeva Clinical Platform. This was a key opportunity we saw for implementing Veeva Study Training.” – Ellen Vanderlinden, Global Clinical Capability Strategy Lead, Bayer
From time-consuming to just-in-time
As well as benefiting sites, Veeva Study Training has changed how Bayer’s study teams plan, deliver, and monitor training. They now automatically assign training by role and geographical location using the training matrix builder featured in the system. Study teams can create training curricula for each study in their portfolio, whether it’s protocol-specific training using documents in Veeva eTMF or non-protocol-specific training, such as Good Clinical Practice (GCP). Veeva Clinical Platform uses the matrix to automatically facilitate training to the relevant study personnel. “Veeva really worked magic by getting the training matrix builder into the system,” says Vanderlinden.
The outcome is the ability to deliver just-in-time training, focusing on what each role needs to know, and when, in order to properly treat patients.
“Veeva really worked magic by getting the training matrix builder into the system.” – Ellen Vanderlinden, Global Clinical Capability Strategy Lead, Bayer
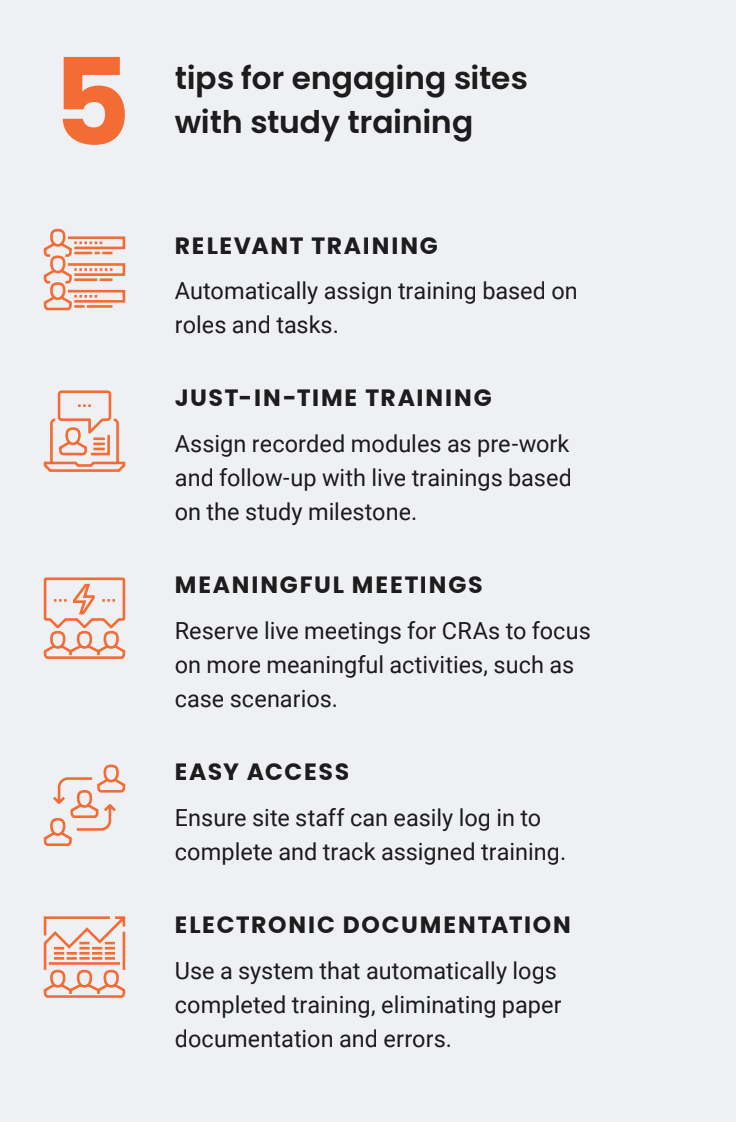
Vanderlinden’s team collected feedback from sites and discovered that most sites begin thinking about training about four weeks before they are ready to enroll. “We want them to be able to focus during this time on training,” says Vanderlinden, adding: “This is where Veeva Study Training is facilitating that just-in-time approach.”
Another manual process alleviated by Veeva Study Training is managing study documentation. Veeva Study Training connects to Veeva eTMF to eliminate the need to manually capture study and site information. “We see Veeva Study Training as a vehicle to support our process from the start of how to deliver training up until getting documentation in Veeva eTMF,” explains Vanderlinden. “The auto-filing back to Veeva eTMF will close the loop, giving us a compliant way to have that oversight.”
A high-quality system for all
A study training platform must meet the needs of several key stakeholders: it should be easy to use for learners, simple to track learner progress for CRAs, and be a reliable tool for sponsors. Vanderlinden was confident in the Veeva Study Training platform, particularly as it is built on a mature learning management system. But she was keen to find out whether the Veeva Study Training platform was meeting other stakeholders’ needs. They shared that they benefit from using VeevaID, which is a login unique to each site user that enables them to access all their Veeva systems.
“They really like the fact that it’s one system for various studies, and that it’s easy to access. They saw the benefit with the onboarding of new staff throughout the study as well.” Based on this feedback, Vanderlinden estimates an almost 100 percent adoption rate of Veeva Study Training among sites.
The increased oversight that the platform provides, along with automated reporting and dashboards, are favorite features among CRAs. Vanderlinden describes the reaction from her internal team: “We’ve decreased the learning curve for CRAs to work with the system and accelerated the study start-up process.”
Continuing training evolution
Reflecting on her team’s journey with Veeva Study Training, Vanderlinden highlights their collaboration with Veeva as a crucial factor for success. “It’s been unbelievably great. There has always been an open conversation, with us bouncing ideas off of each other,” she says.
Looking ahead, Bayer will continue to increase its automation of study training and expand its use of the system to more sites. Vanderlinden is also planning to evolve how training is assigned: “I’m particularly excited about the responsibility aspect of task-based training. In the future, we want principal investigators to be able to assign task-based training, to further tailor it for each individual.”
Learn more about how Vanderlinden’s team uses Veeva Study Training to enable an automated, end-to-end process in a validated system.
More Customer Stories
Explore and learn more
Watch Video
Novotech: Eliminating Silos and Driving Efficiencies with Veeva Study Training

Watch Video
Study Training Without Spreadsheets: How Cerevel Drives Efficiencies and Reduces Risk

Watch Video
Bayer: Streamlining Training and Enhancing Oversight with Veeva Study Training
