Blog
Implementing Study Amendments Without EDC Data Migrations
Jul 03, 2017 | Richard Young
Jul 03, 2017 | Richard Young
Revolutionizing database design with modern EDC
The Challenge in Clinical Trials Around Migrations:
In November 2012, Tufts published its first clinical trial complexity findings and demonstrated how the rising number of protocol amendments hampers our ability to deliver clinical trials. In a Phase III study, they concluded an average of 3.6 protocol amendments, each containing 8.5 changes. That is over 30 individual changes in total.
This number of database changes does not include other changes, such as corrections, updates to procedures, addition of edit checks, etc. If we were to consider the total number of structural and functional changes required, the count would surely be much higher.
Applied Clinical Trials published an article on how the complexity of the study and CRF designs seems to directly correlate with the number of amendments needed in the eCRF. However, it is not the number of amendments that causes surprise here. Rather, it is the fact that for the most complex studies, on average 17 study migrations are required. As we project forward, into the era of adaptive trials, and precision medicine, how will these numbers further increase? Why are “migrations” a big challenge that we need to be especially aware of?
In 2015, Joris De Bondt, SGS, published an approach to amendments that concluded a simple change that took 10 days to implement, and a complex set of changes, much longer.
“Mid-study updates can have limited to wide impact on the eCRF. The typical turn-around time for implementing a limited change, for example, adding 1 existing form in a new visit (folder), is 10 working days.”
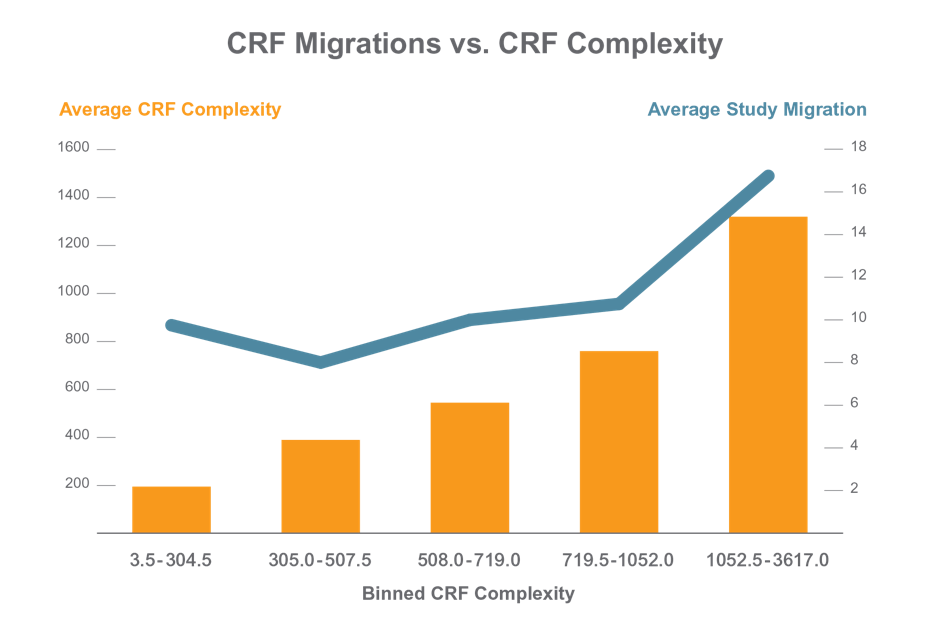
Reference: https://www.appliedclinicaltrialsonline.com/time-another-crf-migration
The key challenge is that in order to make these changes, such as add an additional assessment mid-study, traditional EDC systems need the user to build a new database and physically move the clinical data. To achieve an effective data migration procedure, data on the old system is mapped to the new system utilizing a design for data extraction and data loading. The design relates old data formats to the new system’s formats and requirements. Programmatic data migration may involve many phases but it minimally includes data extraction where data is read from the old system and data loading where data is written to the new system.
After loading into the new system, results are subjected to data verification to determine whether data was accurately translated, is complete, and supports processes in the new system. During verification, there may be a need for a parallel run of both systems to identify areas of disparity and forestall erroneous data loss.
In order to manage this process, it is of course necessary to halt work in the original database, while these time consuming processes are effected. In other words, downtime abounds. Each migration requires study downtime ranging from several hours to several days, causing delays in the study.
Traditional EDC vendors actually advocate sponsors and CROs simplify their eCRFs to minimize downtime, rather than fix the inherent issues in the underlying product design. This results in the execution of sub-optimal study designs, increased costs, and risk to data quality.
This is a situation that underlies the need for a new solution, one that not only simplifies change management but actually embraces it. In an era of precision medicine, we will be making changes more frequently than ever, and we must be able to make and implement changes in real time.
An Alternate Approach
Modern EDC allows for an underlying database design that is as dynamic as the clinical trial itself. This is because the data can be stored in a manner that creates flexibility and removes the need for migrations entirely. Inserting new data collection elements, new pages, new visits, without needing to bring the system down or migrate the entire database would imply that we can simplify the initial build process, and simplify our approach to subsequent amendments. On a page level, we can automatically lock users out of an individual page, for the few seconds it takes to effect that change, and then release the page. Normal service will be resumed in seconds, not hours, or days later. The administration costs will become insignificant and the site will have no downtime due to migrations. In extreme or sensitive examples, the deployment can be scheduled for delivery outside business hours, and the amendment will be invisible.
Taking this further, not only does the build process create EDC, but also creates the site source documents – eSource. This results in one build for both EDC and eSource with one amendment process.
Veeva has delivered the next-generation EDC system, designed to specifically address the migration issue. Vault EDC reduces costs and eradicates down time by implementing changes in near real-time. This is achieved through an architecture designed to support multiple CRF designs and adding/updating only the net changes. Any study instantiated CRFs or parts of CRFs unchanged in design are completely untouched. This ensures that only objects with modified designs are affected. Further simplicity and ease comes from the fact that updates to implement CRFs only require a simple reference change to the new definition. The design yields yet another benefit which allows individual sites and even individual subject casebooks to be updated with no site-level downtime.
Vault EDC uses reference IDs as key to the underlying data rather than use the physical location of the data. Vault EDC can always track the lineage of data points from one study to the next, delivering the advantage of re-use of definitions.
With traditional EDC, a new version of the CRF is considered 100% new version, but with Vault EDC only the delta is managed. Therefore, study migration is obviated and the system can have multiple versions of the case book at any time. Implementation of changes is delivered by “locking out” users on an impacted site/patient/visit/page for the few moments that the change takes – measured in seconds and not days. Vault EDC allows scheduling these changes in advance so they are implemented during non-working hours at each site. Prior to changes being made, an impact analysis report can be run to help review changes and educate sites / study personnel effectively on new and updated requirements. An additional advantage with Vault EDC is the ability to roll back the eCRF to the version used for entry via audit trail. This means auditors can always view the data using the version it was originally recorded in, as well as the current version. Every case book can reference a different definition if need be, so could take this dynamicity to the granularity of the subject level rather than just the study level.
Veeva is delivering a better EDC that will bring a world of clinical trials without migrations and no downtime. This will carry things one step further and ensure that all eCRF changes are seamlessly shared between both EDC and eSource systems.
To learn more on Veeva Vault EDC and how you can get better clinical data faster, visit our resource center.
