
Speeding EDC Build Times
Vertex uses Veeva EDC to speed study builds and expand operational excellence in clinical data management.
Historically, Vertex’s EDC database build times averaged 12-14 weeks, significantly slower than they wanted. The data management team adopted Veeva EDC and began seeing immediate results.
Reduced build
times to 6-8 weeks
Go-lives always before
First Patient First Visit
Highest standards
maintained
Vertex embraced an Agile Design methodology and transformed the way their EDC studies are built. Read the customer success story
Fusing Specifications and Design & a Standards Library Increase Build Efficiency
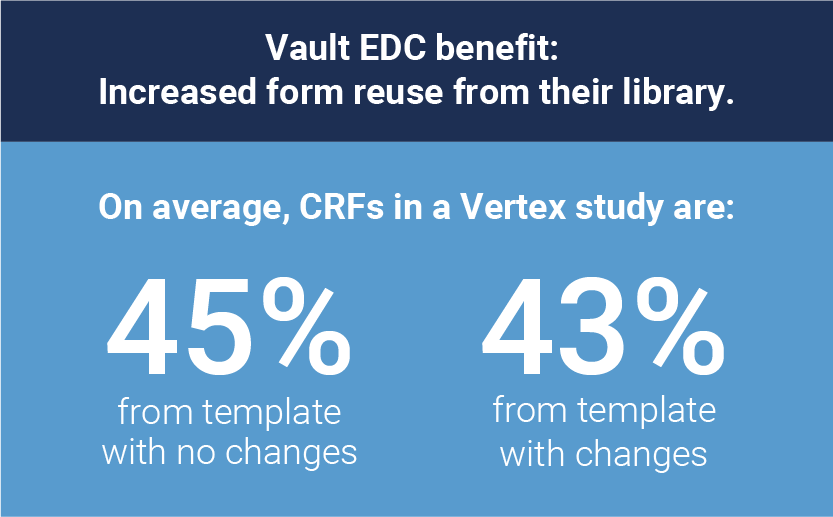
Veeva works directly from study protocols and a database template based on Vertex’s extensive standards library.
- Reusing forms from their standards library increases build efficiency.
- Eliminating the authoring and reviewing of specs, saves weeks of time with each study.
- Conducting live, cross-functional design reviews ensures the correct data is captured and the appropriate checks are run.
Risk-Based UAT Reduces Testing Needed
An innovation in Veeva EDC called the Study Differences Report allows data management to see any additions, omissions, or changes between studies. Using the template study as reference, Vertex no longer performs UAT on forms that were previously tested.
Vertex provides a detailed discussion of current approaches and future considerations for shortening UAT cycles.
Real-time UATs Cut Weeks from Timelines
For the forms that still need UAT, Vertex adopted a live, interactive roundtable approach. Study team members gather in a room while Veeva makes updates to the casebook in real-time.
“We didn’t pick an easy study, and we didn’t expect things to go so fast. We had done three rounds of UAT in two days. Just as soon as we had provided feedback, it was already incorporated.”
Michelle Harrison, Director, Clinical Data Management
