Blog
Safety Track Highlights from 2020 Veeva R&D and Quality Summit
Nov 18, 2020 | jing.to@veeva.com
Nov 18, 2020 | jing.to@veeva.com
This year’s safety track at the 2020 Veeva R&D and Quality Summit focused on streamlining and collaborating. With many people working virtually, making processes easier across teams and partners is critical.
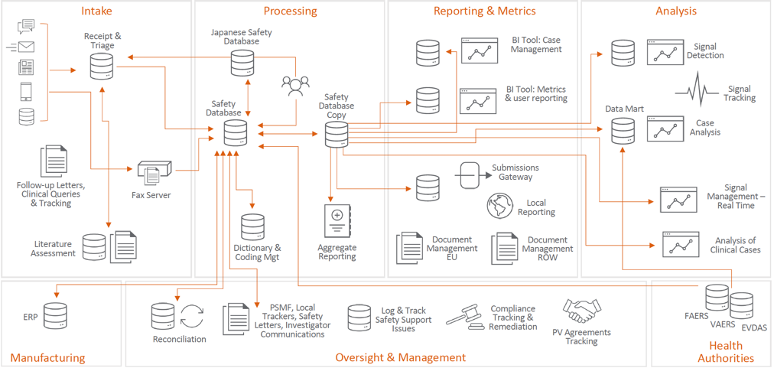
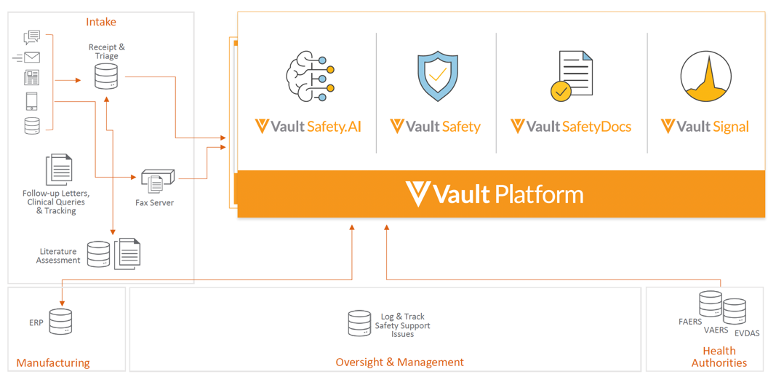
The opening Veeva keynote with Kelly Traverso, VP Vault Safety Strategy, outlined the Vault Safety vision of building a modern and unified solution that provides real-time visibility into safety data. The current pharmacovigilance system architecture is fragmented and, in some cases, outdated. Requiring significant upgrades, it is a strain on resources and budgets.
A unified pharmacovigilance solution simplifies processes and provides better visibility and traceability of safety data.
There was also a sneak peek of the upcoming Vault Signal product. As part of the Vault Safety Suite, Vault Signal will manage signal detection through risk evaluation and mitigation.
G1, a Vault Safety customer, discusses how integrating Vaults can further streamline pharmacovigilance processes. Their Vault MedComms and Vault Safety integration will allow adverse event data collected in the call center to be immediately visible to the safety team – eliminating duplicate data entry, allowing tracking of adverse events back to the original call, and reducing data reconciliation. It is “a huge step forward in terms of efficiencies for case processing and reporting of safety data,” said Richard Dyer, senior information technology solutions architect, G1 Therapeutics. Vault Safety is also making it easier for G1 to work with their CRO as all parties have direct access to case data. With Vault Safety, the G1 safety team does not have to wait for information from their CRO and are able to easily find and address erroneous, duplicate, and other data issues.
CROs provided their perspectives on collaborating with biopharma companies and Vault Safety in the panel session. With recent changes in work environments, ease of working across geographies became important. Catalyst and Arriello found Vault Safety easy to use for administrators and end users. They are able to easily onboard clients – anywhere – and share information and collaborate. Bringing on existing Veeva customers of other Vault applications was even faster as they are familiar with the solution. The system is also flexible said Catalyst’s application admin, with “high configurability without high complexity,” according to Lisa Hornick, M.D., chief medical officer, Catalyst Clinical Research.
There was a lot of interest in connecting Vaults and Veeva provided an overview on the approach. We looked at how connections are enabled by Veeva Vault Spark and potential use cases for integrating Vault Safety with the following Vaults:
- Vault eTMF to streamline management and distribution of safety letters
- Vault EDC to provide real-time visibility of adverse events collected from sites
- Vault CTMS to eliminate duplicate data entry. Vault Safety will share product information and Vault CTMS will provide visibility into trial information such as sites, study arms, and investigator information
- Vault MedComms for automatic transmission of AE data for processing
Over the two-day event, best practices regarding implementation and migration were shared by NNIT and Veeva. NNIT has extensive Vault migration experience and discussed essential ingredients for success, and considerations for migrating safety data. The Veeva services presentation focused on implementation best practices and explained their holistic approach, implementation risks, and ways to mitigate them. They also reviewed implementation accelerators such as best practice methodologies, templates, and validation kit.
If you missed one of the sessions or would like to see it again, please click here to view the recordings.