Vertex Data Management Team
Cuts EDC System Build Times by Half with Veeva Clinical Data
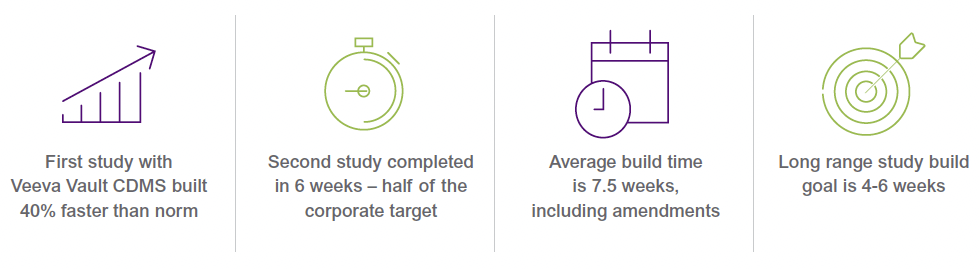
The data management team at Vertex has an impressive track record for operational excellence. In their trials, sites typically enter data within 48 hours of the patient visit, and Vertex locks their data—all study data—within 15 to 18 days. Improving the efficiency of study builds is their next step towards end-to-end operational efficiency. And they’re succeeding. Their first early phase study in Veeva Clinical Data was built in just eight weeks, 40% faster than their historic norm of 13 to 14 weeks. The second was completed in only six weeks, cutting their original corporate target of 12 weeks in half. Today, the target for completing study builds with Veeva EDC is six to eight weeks. The long range goal is for all study builds to be completed within four to six weeks.
Success Highlights
The Challenge: Lengthy Time to Go-Live for EDC Builds
Historically, Vertex’s builds took an average of 13-14 weeks. These timelines were in line with industry norms, especially for the complex studies Vertex was running, but much higher than they wanted. As a result, there was a risk to a database going live after first patient screened and thus the sites would need to wait to enter data.
A major contributor to the long timelines was the back-and-forth with their vendor that took place during the casebook design and again for user acceptance testing (UAT). At Vertex, the process for UAT was the more painful of the two. Historically, the vendor completed a UAT and sent the casebook to Vertex. Data management waited for comments from the study team before returning the aggregated feedback. A revised casebook was sent to Vertex for the next round of UAT. This “ping-pong” approach was time-consuming, with each round taking one to two weeks.
Stakeholders outside of data management would often make suggestions and request changes without understanding the downstream implications on the EDC. This required offline conversations between data management and other study team members, slowing the process even more.
When Vertex executives set a Development-level goal to speed studies from protocol finalization to data coming in, the data management team knew that speeding study builds would become a top priority.
The Solution: Veeva Clinical Data
Vertex partnered with Veeva and set an initial target for build times of six-to-eight weeks, and a long-term goal of just four-to-six weeks for early phase studies. Condensing 13-14 weeks into eight, and eventually four was a daunting task, and would take a combination of modernized EDC functionality and improved processes.
Working with Standards
Prior to the first study, Veeva services built a casebook template based on Vertex’s extensive standards library. Using the templated Case Report Forms (CRFs) greatly improved build efficiency; all but one of the forms for the first two studies were drawn from the template study and modified as needed.
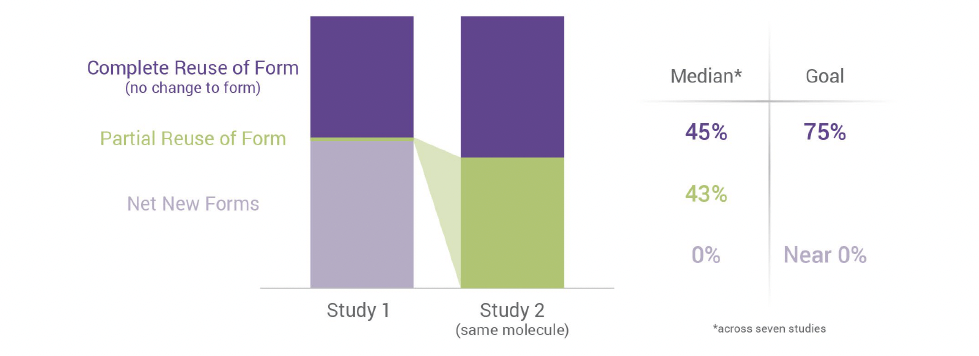
Diagram 1. “Partial reuse” or modifying the template study forms increased from <1% to >48% between the first and second study of one molecule.
Spec-less Design
Vertex does not provide Veeva with specs for building studies. As part of an Agile Design methodology, the Veeva team interprets the protocols and draws from their libraries to generate real screens in a sandbox environment of Veeva EDC. The teams then meet face-to-face for an interactive design review meeting with clinical, stats, data management, and other key contributors.
“It is easier to provide constructive feedback when looking at actual screens than it is when reviewing a traditional spec. It is a very efficient process and the quality of reviews has gone up.” – Michelle Harrison, director of clinical data management
An official spec is created at the end of the process in the form of a system-generated spreadsheet called the Study Design Specification. Everything that exists in a traditional spec is included plus more. The Study Design Specification is used for sign-off and any subsequent changes are captured in the Study Differences Report—another form of system-generated documentation.
“The protocol is your spec. Everything that needs to be analyzed is in the protocol. Veeva works from the protocol, just like they would from a spec. We’re just eliminating a big time consuming step in the middle.” – Vikas Gulati, executive director of clinical data management and metrics
Efficient Build Tools in Studio
There were a few aspects of Studio, the design environment within Veeva Clinical Data, that helped reduce build times.
- Field reuse – In addition to reusing forms from the template study, Veeva also reused individual fields, such as informed consent dates. Once those were built, Veeva reused the field across multiple forms.
- Dynamic items – Certain fields are dynamically included based on prior answers, such as adding questions about child-bearing potential if the subject is female. Those fields wouldn’t be included for male subjects, which reduces the number of edit checks needed.
- Streamlined date collection – Historically, Vertex would include date-of-visit fields on their CRFs. With Veeva Clinical Data, that information is already tracked and known as the “event date,” and isn’t needed on the CRFs.
Relevant, Real-time, and Risk-based UAT
When it came time for UAT, the data management team implemented Veeva’s live, interactive “roundtable” approach. By having all stakeholders in the same room, the team is able to discuss and provide conclusive feedback. Behind the scenes, the software is updated in real-time. These real-time updates are enabled by a modern, flexible architecture. In Veeva Clinical Data, case report forms are maintained and managed separately from the data they collect. This allows changes to the forms or their rules to be displayed immediately in the user interface. In one example, Vertex and Veeva completed what would have been three separate rounds of UAT in only two days, cutting multiple weeks of waiting out of the process.
“Live UAT updates are a game-changer,” noted Gulati. “By providing feedback, fixing problems, and testing updates immediately, we can eliminate three to four weeks from our timeline. This is a big departure from our historic approach.”
More complex changes to the casebook that cannot be completed within the live UAT conference are documented in a shared request-log that both Veeva Clinical Data services and the Vertex data management team can access. While the Veeva services team is revising the database within the sandbox development environment, the data management team has full visibility into the sandbox and Veeva’s progress. The Vertex team approves each update, or notes additional requests within the shared tracking log, creating a close collaboration between the organizations.
To reduce the sheer number of items checked during UAT, Vertex adopted a risk-based approach which eliminates testing for forms and fields that were previously tested and haven’t changed. The Study Differences Report within Veeva EDC enables the risk-based approach by documenting any and all changes between two studies. Vertex uses the report to identify everything in a study that differs from their templated standards and only test the new or changed elements. This approach reduced the amount of time and effort needed for a recent UAT by 50%.
Prior to working with Veeva, their UAT cycles would last multiple weeks. The UAT for their most recent study was completed by two individuals in just two days.
The Bottom Line: Improved Build Times with Veeva Clinical Data
Vertex embraced change and transformed the way their studies are built. Using Veeva Clinical Data and Agile Design processes, they have cut their average build times for early phase studies in half while maintaining their high standard for quality. Vertex plans to conduct nearly 20 studies this year using Veeva Clinical Data and over the next two years will continue shortening timelines until they reach their goal of a four to six week build.
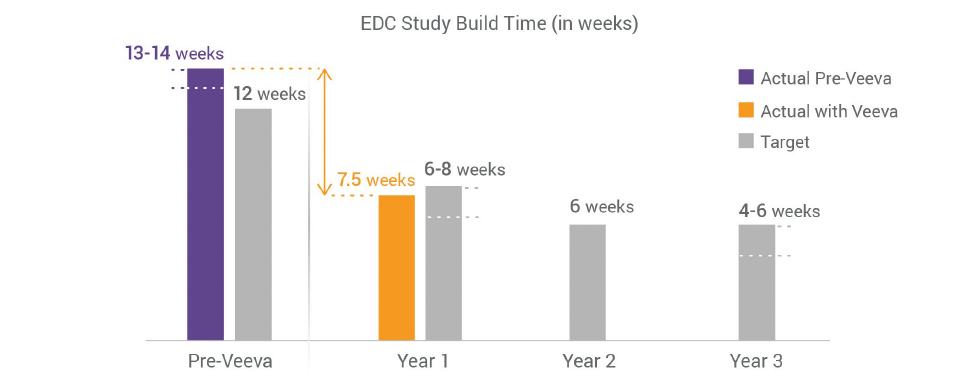
“On average, our build times with Veeva are seven and a half weeks–and we’ve had some major amendments in those studies. Even with putting things on hold, all our studies have gone live before First Subject First Visit and that speaks to Veeva’s speed and agility.” – Michelle Harrison, director of clinical data management




