Article
Using Benchmarks to Speed and
Scale Life Sciences Content
THOUGHT STARTER SERIES
The pressure to deliver more relevant and personalized content never stops—and the expectations to shorten the time to market are growing. On top of that, life sciences content is getting increasingly complex, with a greater need for personalized content that is scalable and interactive.
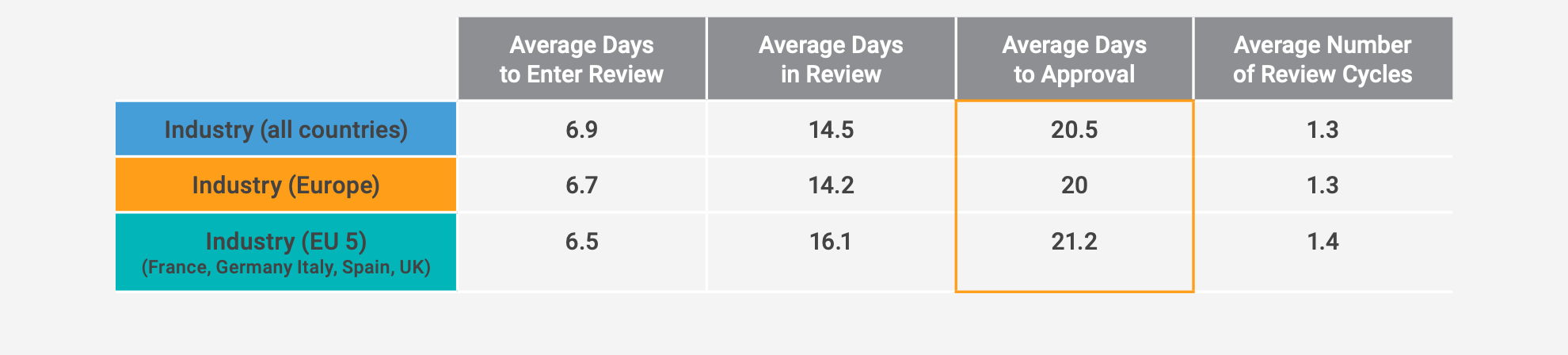
Throughout the content generation pipeline, obstacles can delay getting that content to your stakeholders or in front of your audience. This situation happens most often at the content review and approval stage. In the European market, it can take 20 days on average to get content approved, and the average number of review cycles through medical, legal, and regulatory (MLR) review is 1.3.1
From a regional standpoint, Europe is on par with the global industry’s performance, but a deeper look at the benchmark shows significant differences between European countries. In terms of volume, the benchmark shows that more than half of the content is created within the EU5 countries (France, Germany, Italy, Spain and the U.K.). It also pinpoints significant differences between countries in terms of time to market, illustrating the European challenge to adapt to different regulatory constraints. For example, it takes significantly less time to approval in Germany (on average, 15.4 days) than in France (27.2 days).
A deeper dive at the benchmark at the company level indicates that some organizations have been able to trim the average days to approval by more than 35% and limit review cycles to slightly more than one.2 These improvements don’t require a complete overhaul of processes. Instead, you can refine your content generation pipeline with simple steps to:
- Use benchmarking for guidance
- Speed up content production with better processes
- Reduce review cycles
- Set rules to increase reuse
CONTENT METRICS DATA FOR 2023
Drive your performance with industry benchmarks
Metrics are crucial to draw insights and continuous improvement from your entire content management process. Whether you’ve had comprehensive metrics in place or are starting to measure critical milestones, even month-to-month comparisons can reveal opportunities for improvement.
The right benchmarking data can give you the confidence to shift resources to a weaker part of the process or identify quick fixes that deliver significant returns. For example, if you notice that your review cycles have increased or stalled in MLR, you can focus your improvements on that stage. Your metrics can also identify where things are working, giving you best practices for use in another part of the content process.
Establish your content benchmark matrix
To engage a continuous improvement mindset across the company, it may help to drill down into the metrics through a four-part matrix: global process, best practices, content type, and process deviations. In each, content metrics can help to identify bottlenecks and illuminate the best practices that can be adopted. It can also help you to gather information on comparable companies, content types, regions, or therapeutic areas, and fuel a healthy competition between teams for content excellence.
For example, benchmarking might point out that the process for approving corporate communications or training materials is very effective but is a bit behind the industry on email and digital sales aids. It also helps a company to analyze deviations from a process. For example, it could show that the review time for a particular kind of content in a region is faster not as a result of greater efficiency but because the content team there is not following your mandated process.
Accelerate content production with better processes
You can set up standard operating procedures (SOPs) that the team can follow as a process roadmap. Bring contributors from across the organization who impact content management into the conversation, including commercial and operations teams and agency representatives. This way, you’ll develop meaningful SOPs with buy-in from those who need to follow these guidelines.
Once the process guardrails are in place, bring on a process manager who can own the effort. They’ll ensure that each process milestone is met and manage issues. More importantly, they’ll spearhead the review process, making sure essential people in the organization review content.
If it’s possible, consider developing specialized team responsibilities. For instance, assign a team member to work exclusively with the MLR team. They’ll develop MLR expertise and even help standardize MLR review comments that can inform future content generation.
Track performance with agencies
Content flows to and from agencies at a regular pace. You may rely on an agency of record (AOR) to drive strategy and develop core messaging with each brand in mind. That smooth flow can be interrupted when agency content doesn’t move as quickly through the review process as content from the internal team. And for some of these agencies, staff turnover and lack of onboarding can contribute to slower review times.
Rather than engaging only when there’s an issue, set up an “agency operations” function or simply a quarterly review to help manage agency performance. You’ll be able to benchmark agency performance regarding financial management, how they operate in the MLR process, training, and onboarding.
Reduce review times and cycles
Reducing the number of review cycles and the time copy spends in MLR is one of the surest ways to move content quicker through the pipeline. Getting there requires a little pre-planning and working more closely with the MLR team.
- Raise content issues early: Set up opportunities early in the content development process to vet content thoroughly. Bring a complete mix of people to the table who are likely to raise concerns or challenge the content. Also, consider adding MLR teams earlier for feedback before content moves through review.
- Identify dedicated reviewers: If you have the capacity, set up a core review team that includes a medical reviewer, a legal reviewer, and a regulatory reviewer. Having these subject area experts weigh in on the language and topics can improve review time.
- Set review timeframes: Thread throughout your process clear timeframes that underpin each SOP. Work with the team to identify the number of days allotted to each timeline stage. For instance, provide a specific number of review days and modify it for expedited projects. The team likely works with various projects, so set timelines to match content size—providing shorter timeframes for smaller word count with no references versus an ebook requiring several citations, or a flashcard versus a deck for your health economics team.
- Consider final use: Whether it’s posted online or added to a print piece, the content might need to move through different channels based on how you deploy it. For example, events with virtual booths can raise unique content-sharing concerns. In this case, you could standardize virtual booth processes and displays to keep review paths predictable.
Set the rules and measures for content reuse
The benefit of using derivative content is that you can seamlessly lift it from approved core content—it has made it through reviews and is ready for a new use. But it isn’t always that simple.
In many cases, MLR reviewers need another pass at approving derivative content. That’s due to the likelihood that content drawn from the core can lose the context that drove approvals from the original review. The derivative content may lose its full meaning or alter compliance language without that context.
One way to avoid drawn-out second reviews of the same content is to put business rules in place. These rules attach claims or relevant core content to modular, pre-approved content. For example, if your copy focuses on efficacy, you may require an additional copy on safety or add a link connecting to a claim that supports that efficacy language.
Key Takeaways
- Use benchmarks
Develop insights on your content management efforts by setting up metrics to measure both your success and opportunities for improvement. Compare with industry benchmarks to identify gaps and areas for improvement. - Standardize processes from global to local
Equip your team with the proper process guardrails to move quickly through approvals and deployment. Adjust your global process to meet the regional and country-level differences. - Include MLR early
Speed approvals by bringing the MLR team to the table earlier in the content development process. Partner with reviewers to build templates and develop standards for reviews. - Ensure reuse capabilities
Identify the types of derivative content that might need additional approvals and build rules to help streamline the reuse process.
1, 2 Veeva Content Metrics data, 2023
Read the 2022 Veeva Pulse Content Metrics Report
for data and insights to improve your content strategy
John Oxley
Business Consulting Practice Manager, Veeva
Orestis Ntarlas
Senior Business Consultant, Global Commercial Content, Veeva
About Veeva Business Consulting
Veeva Business Consulting combines commercial and medical expertise with Veeva’s proprietary data and technology to deliver better business-focused solutions for our customers. Our team of experts offers a suite of advisory offerings, including launch readiness, digital acceleration, and content optimization, all supported through unique HCP insights and analytics.
To learn more, visit: veeva.com/eu/business-consulting.
