eBook
How to Choose the Right eTMF Model
For many emerging biopharmas and biotechs with limited resources, outsourcing trial master file (TMF) management to contract research organizations (CROs) is a given. Partnering with CROs allows smaller sponsors to reduce manual document reviews and improve their TMF quality without increasing overhead. However, relying solely on a CRO to manage all TMF activities can limit your visibility into TMF content and processes. It also poses a regulatory risk for documents such as oversight plans, risk management plans, and internal SOPs.
It’s often difficult for smaller companies to choose an operating model that works for their organization’s size, budget, and trial portfolio. Sponsors sometimes start with a hybrid model where their team manages their documents in their own eTMF, and the CRO team manages the remaining trial documentation in their CRO-owned system. The CRO then transfers documentation to the sponsor-owned eTMF at the end of the trial. In this model, the sponsor ensures their documents are inspection-ready without managing all trial documents in their own system. Later, as a sponsor’s trial volume and clinical team grow, they can consider, expanding the use of their eTMF system and inviting CROs to work in it as collaborators.
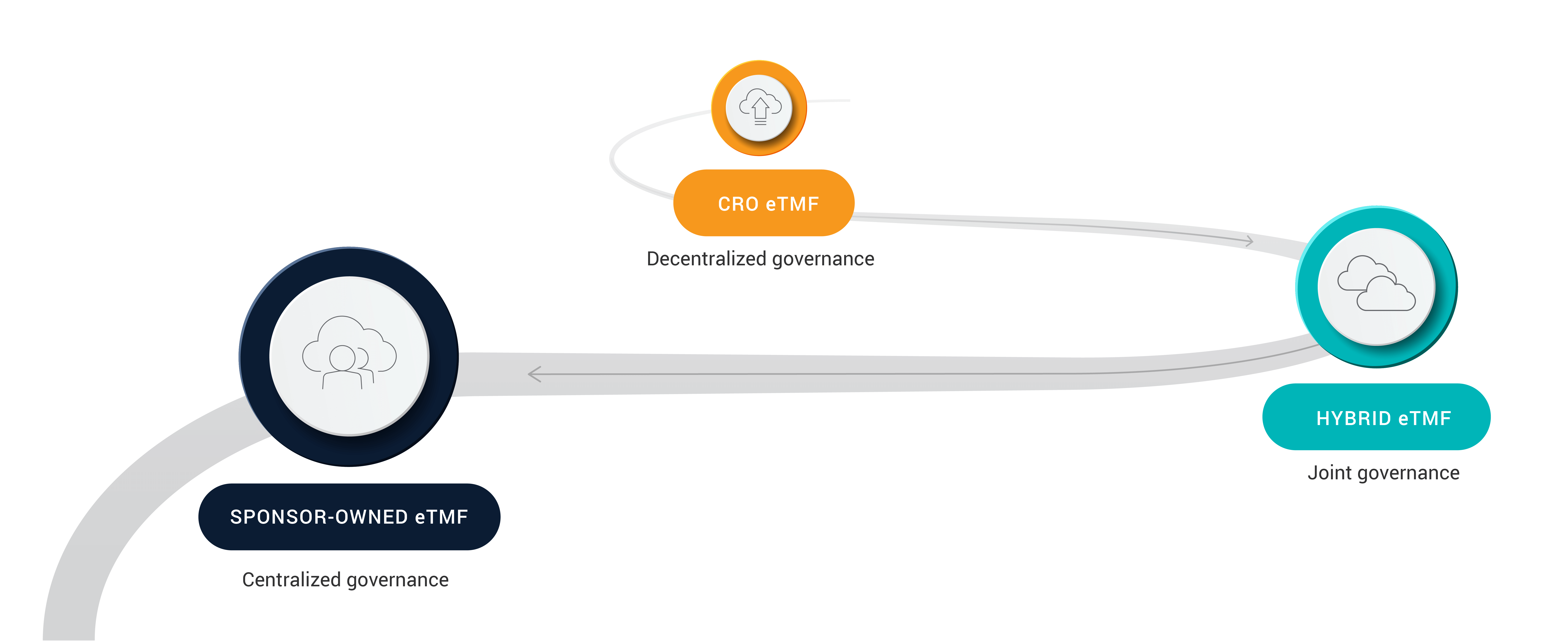
This three-part guide walks through several TMF operating models that drive inspection readiness, including:
- Best practices for implementation
- Signs you’re ready to own your eTMF
- Insights and tips from sponsors and CROs
Table of Contents
Model one: Hybrid sponsor and CRO eTMF
Going hybrid
A hybrid sponsor and CRO eTMF model is a good starting point for growing biopharmas. “A CRO generally has its own systems and SOPs geared toward their processes,” says Ruth Ruffieux, head of clinical operations at global biopharmaceutical company Alvotech. “As a small biotech, it’s difficult to ask a big or mid-sized CRO to stop doing what they’re doing and work in our system. That was one of the main reasons why we selected this model.”
You can also assess whether to use a hybrid model on a study-by-study basis. OM Pharma, a global Geneva-based biopharmaceutical company, implemented Veeva Vault eTMF in 2022. The company currently has five studies running from Phase I to Phase IV and works with two CROs. OM Pharma chose a hybrid eTMF model for one of its Phase IV studies spanning one country and 50 sites. That way, the CRO could manage the bulk of the trial in its system and help OM Pharma conserve its resources.
“We chose this model because we had a large number of sites, meaning a greater number of documents to manage,” explains Nelson da Silva, eTMF specialist and clinical trial associate lead at OM Pharma. Another key factor in the company’s decision was that the CRO on this study already used Vault eTMF and Vault CTMS. In a hybrid model, the CRO can more effectively run the study in its connected systems and transfer TMF documents at the end of the study to OM Pharma’s eTMF system.
Splitting responsibilities
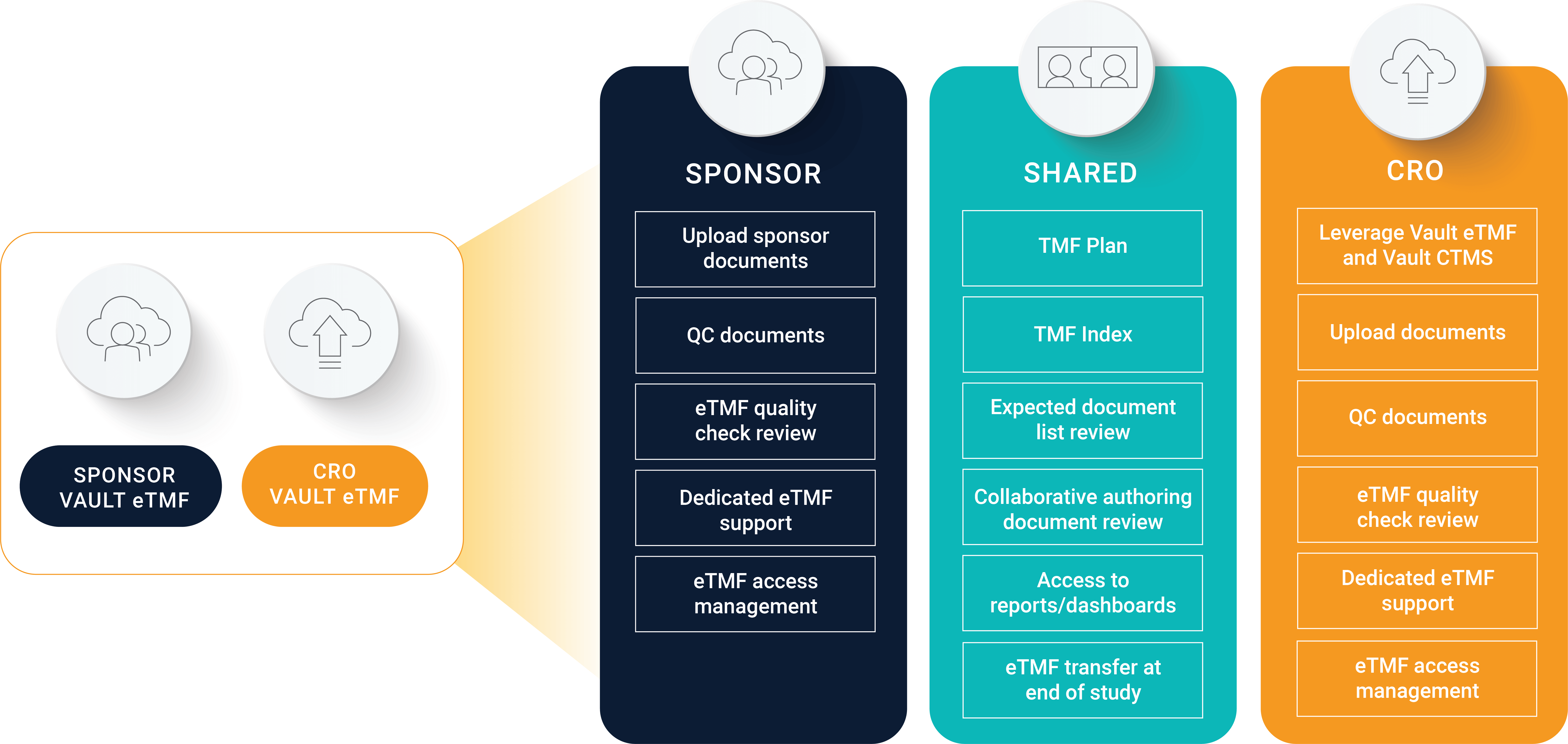
In a hybrid model, the split of responsibilities between you and your CRO can vary. For example, the OM Pharma team maintains and QCs their documents in Vault eTMF, and its CRO does the same in its own eTMF system. They define responsibilities at the outset of the study in an agreed-upon TMF plan, and OM Pharma provides oversight throughout the course of the study.
“Our CRO does not have access to our Vault eTMF, but we have access to their eTMF. As the sponsor, it’s important to us to have that direct access so we can do proper TMF oversight,” da Silva says. OM Pharma accesses the CRO’s reports and dashboards on an ongoing basis to check on completeness, and the CRO transfers documents at the end of the study. In this hybrid model, OM Pharma can give more responsibilities to the CRO, like eTMF reviews and access management, rather than managing those tasks on the sponsor side.
Increasing collaboration
For da Silva, the greatest benefit of the hybrid TMF model is increased collaboration with CRO partners. He credits this improvement to the Collaborative Authoring feature of Vault eTMF. Collaborative Authoring connects Vault to Office 365 and allows multiple users to edit Office documents in a shared library. They use this feature as external users in the CRO’s system, and vice versa in their own system. “Now, we work together with our CRO and don’t have to go outside the system to review documents,” says da Silva.
“Being inspection ready means consistently keeping track of document reviews, changes, and version history.” – Nelson da Silva, eTMF specialist and clinical trial associate lead, OM Pharma
Implementing a hybrid model reinforced da Silva’s view of what it means to be truly inspection-ready. “Inspection readiness isn’t just about having your final documents uploaded into your eTMF,” he explains. “Being inspection ready means consistently keeping track of document reviews, changes, and version history.”
An associate director of clinical operations at UK-based CRO Simbec-Orion agrees. “We've all lost documents in our inbox in the past. If documents never leave the system, then your system is always inspection-ready,” he says.
The benefits of a hybrid sponsor and CRO eTMF model include:
- The sponsor maintains confidentiality and ownership of certain documents in the sponsor eTMF.
- The CRO works in their eTMF with links to other CRO-owned systems.
- The CRO documents are transferred to the sponsor when the study is complete and archived in the sponsor eTMF.
Read on to part two of this guide to know when your organization is ready to have CROs work in your system.
Model two: Sponsor-owned eTMF
As your trial volume and clinical team grow, you may consider expanding the use of your eTMF system and inviting CROs to work in it as collaborators. Bringing your eTMF in-house might feel daunting. But, it may be the best option to meet your company’s needs as it matures. “When you’re a smaller sponsor working with a big CRO, it might be hard to negotiate with them, but it can be done,” says Pawel Rucki, clinical business consultant at BASE life science.
There are a number of factors that influence this decision, including growing trial volumes and clinical teams. For Ferring, a Swiss multinational biopharmaceutical company, inspection findings pushed its clinical operations team to make a change. “We were struggling to prepare for audits and inspections, or find documents on time with TMF documents across multiple systems and locations. Our TMF process was simply not working efficiently,” explains Christian Bruun Sanders, Ferring’s process and systems manager.
“We were struggling to prepare for audits and inspections, or find documents on time with TMF documents across multiple systems and locations. Our TMF process was simply not working efficiently.” – Christian Bruun Sanders, process and systems manager, Ferring
argenx, a global biopharmaceutical company, struggled with similar challenges. “In recent inspections, it could take us up to four hours to get a document because we would need to identify where it was stored and whether we had direct access,” remembers Melissa De Swaef, argenx’s head of clinical operations processes and systems. “We want to eliminate these steps with a single source of truth and CRO access.”
Building a business case
The first step to bringing your eTMF in-house is building a strong business case. For example, argenx started by weighing the costs and savings of making the switch from hybrid to in-house. The costs include implementation, user training for CROs, and system maintenance. However, an in-house system also reduces end-of-study migration costs, cuts time spent preparing for inspections, and saves resources. “Our eTMF end-users were also really looking forward to having a single system. Even something as simple as collecting an electronic signature was difficult because each organization had its own software,” says de Swaef.
Ultimately, argenx’s financial models predicted that an in-house eTMF would provide a return on investment (ROI) of 100% over five years. The shift would allow the company to eliminate all end-of-study migrations, reduce audit and inspection preparation time, and manage documents more effectively across partners.

Bringing eTMF in-house
Bringing your TMF operations in-house can also be a good time to reevaluate and improve existing processes. Ferring used the opportunity to harmonize its TMF activities globally. “We previously had different ways of working with TMFs in each region. Now, we have standardized processes in a single system where everyone has oversight of documents from when they’re first drafted to when they’re archived,” says Sanders. This continuous oversight simplifies audits and inspections for Ferring. “We give auditors access to Vault eTMF and assign them to a specific study,” he explains. “It’s so much easier for them to review all our TMF documents.”
Sanders’ team also created a new TMF manager role to maintain consistent TMF quality throughout their trials. Currently, Ferring has four TMF regional managers. “The TMF managers also act as ‘super users’ of Vault eTMF. This helps streamline communication between my team, as system managers, and our eTMF end users,” he says.
Providing CRO access
When leveraging your own eTMF, you should have plans in place for providing system access and administering training to your CROs. For example, the IT team at a global Danish biopharmaceutical company runs joint quarterly reviews with its CROs to ensure they have access to the right studies. This helps the company account for turnover when managing access and training.
Currently, the biopharma company has about 500 external users in Vault eTMF. “Sometimes, people have left [the CRO] and need to be removed from the system. We needed to develop better access management processes,” says the company’s clinical process manager. “It’s been really helpful for us to get together with our CROs to make sure our access lists are accurate.”
Training is also pivotal to ensuring CROs are bought into your eTMF. “Having training sessions in our CROs’ learning management system has cut down on manual efforts for us because we’re not sending training to each new user. When our CROs onboard a new team member, they have access to them right away,” explains their clinical process manager. This training is comprehensive, including everything from standard system navigation to detailed explanations of new features or index changes.
Ultimately, when designing training, it’s important to view your CROs as your partners. “CROs are not just something you need to oversee. They are there to help you, and that's how you need to treat them,” says Rucki. “They will be using your eTMF more than you, so you need to make sure that training is effective and meets their needs.”
The benefits of bringing eTMF in-house include:
- All TMF documents are in one place.
- The sponsor’s single global system enables increased harmonization and collaboration.
- The sponsor improves day-to-day oversight and inspection readiness.
CROs – Read on to part three of this guide to learn more about improving collaboration in hybrid and sponsor-owned eTMF models.
CROs: Driving trial success in any eTMF model
Nowadays, more and more sponsors are bringing their TMF operations in-house. Although this model is increasingly popular, it can also pose challenges for CROs. CROs may lose efficiencies in their ecosystems, such as eTMF to CTMS connections, when they use a sponsor’s eTMF. CRO users are also trained on and familiar with their own systems.
“If a sponsor is giving you access to their system, they are responsible for training, processes, and oversight,” explains Pawel Rucki, clinical business consultant at Danish consulting company BASE life science. As a CRO, you will likely face both hybrid and fully in-house TMF operating models. The following best practices will help you succeed regardless of the operating model.
Embracing hybrid eTMF
In some studies, UK-based CRO Simbec-Orion uses a hybrid eTMF model with its sponsors. That is, the company’s clinical team works within its own platform and transfers documents to its sponsor’s eTMF at the end of the study.
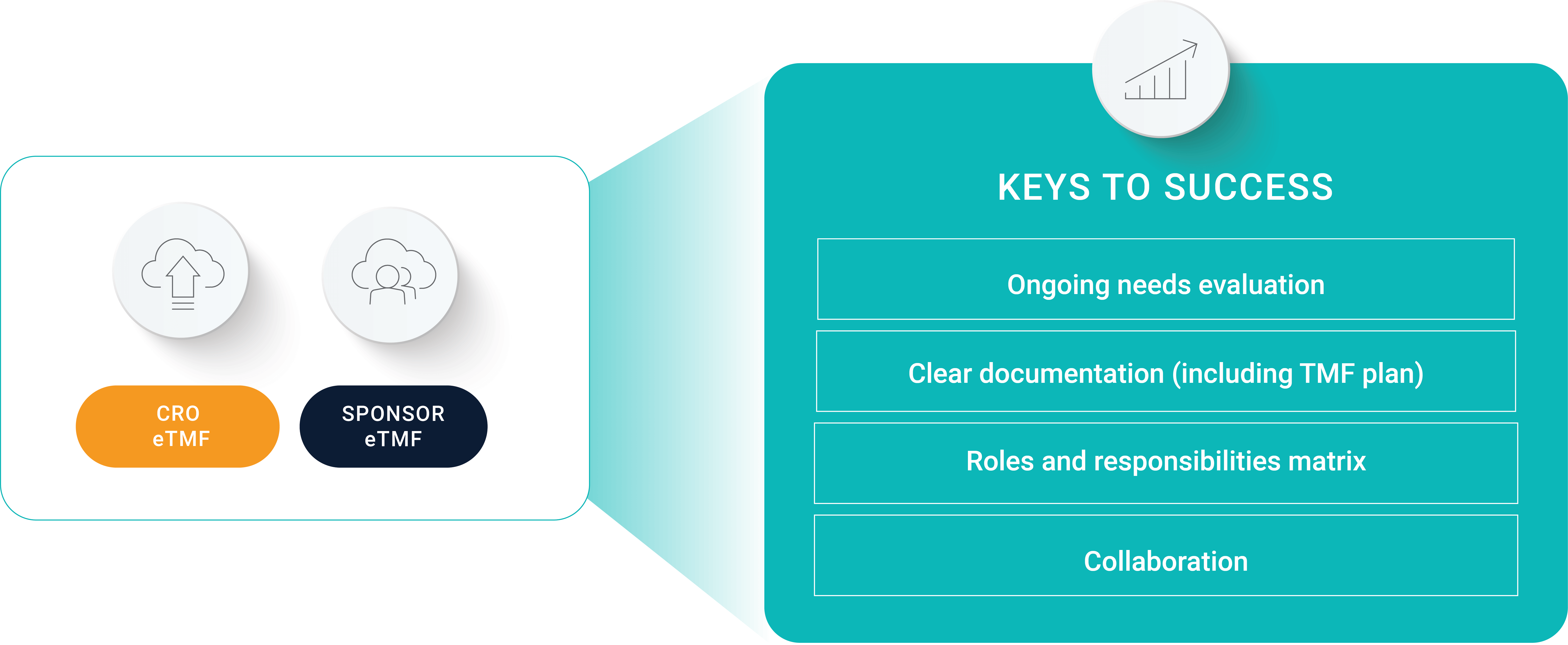
Simbec-Orion’s associate director of clinical operations stresses that early alignment is at the core of trial success. “Make sure you define your responsibility matrix. That way, you can understand who’s responsible for what, and where certain documents are going to live,” he explains. It’s also important to consider your sponsor’s needs. “Collaboration is key. Even though you’re working within your own systems, a sponsor is still going to want to access yours to ensure that they have oversight over both.”
Establishing KPIs
Regardless of the operating model, Rucki advises CROs to define KPIs and metrics early in their working relationship with sponsors. “Every KPI is a metric, but not every metric is a KPI,” he says. KPIs are more strategic, aligned to an organization’s goals, and easily measured. To ensure that the TMF stays on track throughout the study, some CROs and sponsors put KPIs in their contractual agreements. For example, if a site does not recruit as many patients as the principal investigator promised, it may become a contentious issue. Rucki cautions that a sponsor may think the CRO is responsible in these situations, so it’s important to discuss these KPIs early.
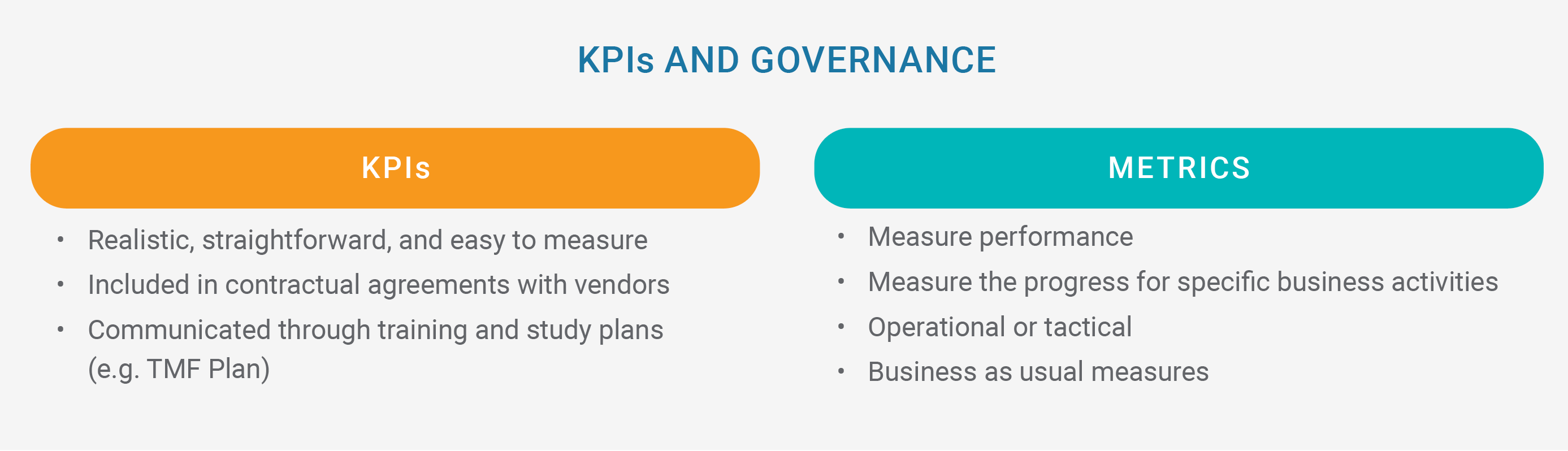
Some KPIs and metrics to consider tracking include:
- Clinical quality measures like document QC rejection rate, post-QC document quality issues, and TMF health
- Timeliness measures such as document finalization and time in QC
- Completeness measures like expected document lists (EDLs) and periodic TMF reviews
Managing sponsor-owned eTMF
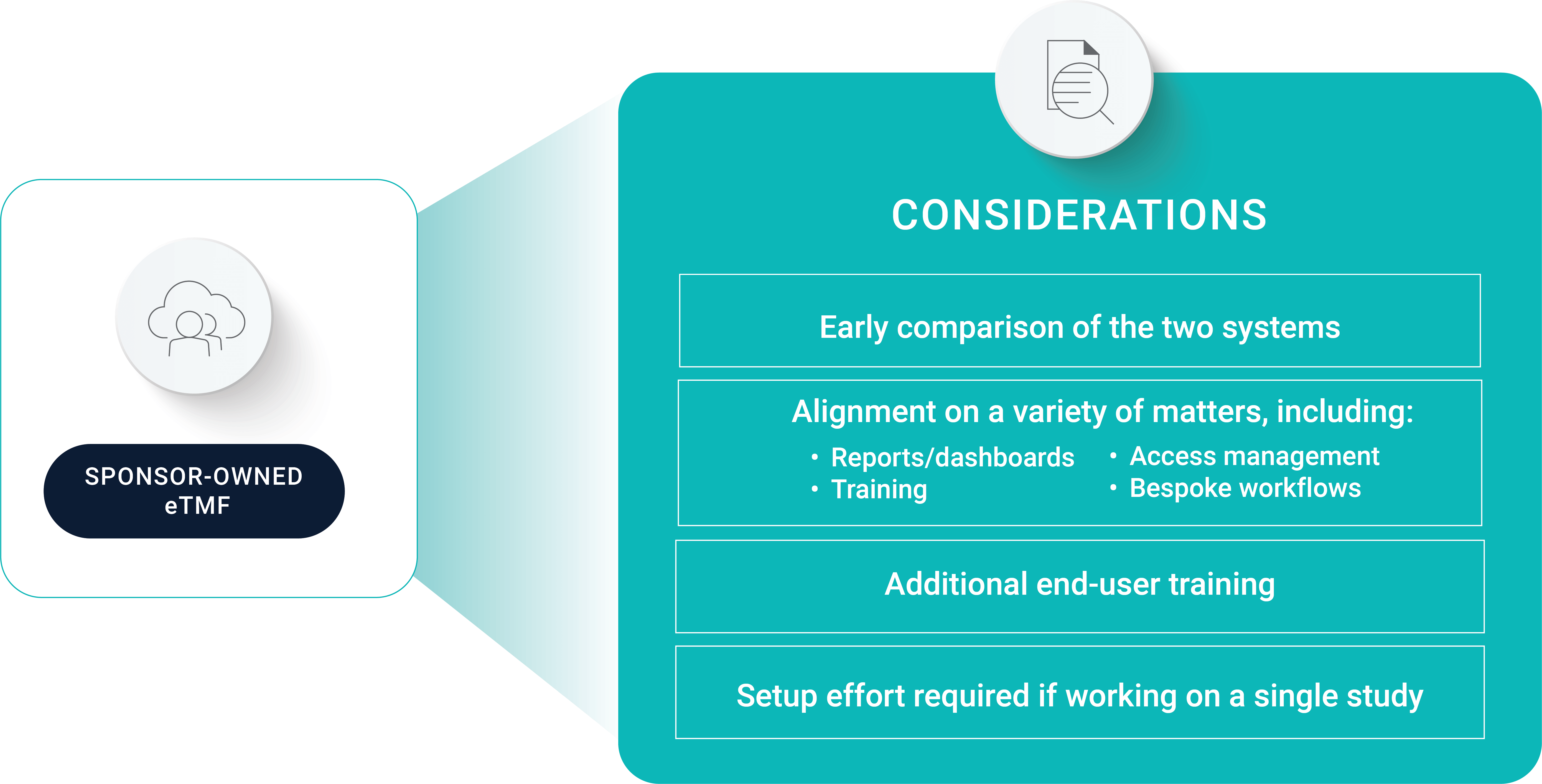
When working in a sponsor’s eTMF, Simbec-Orion’s associate director of clinical operations cautions CROs against leaving eTMF as an afterthought. “There are so many moving parts during study start-up, and it’s easy to let eTMF slip. But, if you dedicate time in a study’s early days to get your TMF ready, it will lessen challenges in the long run,” he says.
To keep TMF top of mind, the Simbec-Orion team schedules an eTMF kickoff meeting at the start of every trial to align early on key decisions. They start by comparing their eTMF system to the sponsor’s to see if there are any differences. If one system has bespoke configurations that the other does not, this may cause end-user challenges. For example, Simbec-Orion has a custom workflow in Vault eTMF to record that users have read documents in the system. “It’s important to make sure the two systems match up and the processes aren’t going to be affected,” says the associate director of clinical operations. If there are differences, sponsors need to train CROs on how to work around them.
Next, make sure you discuss the administrative elements of using a sponsor’s eTMF. “We ask our sponsors if we can get our standard dashboards and reports from their system,” says Simbec-Orion’s associate director of clinical operations. Another important component is agreeing on whose SOPs to use. “SOPs are generally written with certain systems and configurations in mind. So, if the configurations don't match, that could impact SOPs and processes,” he says.
Since you will be working in the sponsor’s eTMF system, it’s important to come together with your sponsor to design a training program that fits your organization’s needs. For example, some CROs will ask sponsors to deliver training on a certain timeline. As the trial progresses, multiple eTMF configuration changes may require additional training. You can ask your sponsor to bundle training or provide a summary of changes to save time.
Ultimately, whatever model you’re working in, ensuring inspection readiness is about empowering your teams to make TMF a priority from the start. “If it's a priority from day one, it's going to be so much better and easier for you further down the line,” says Simbec-Orion’s associate director of clinical operations.
Learn how a sponsor collaborates with CRO partners to improve oversight and inspection readiness.
