Blog
How To Increase Sponsor Compliance with Vault eTMF While Outsourcing
Sep 06, 2024 | Pinar Bérénice Benét
Sep 06, 2024 | Pinar Bérénice Benét
The new ICH (E6)R3 regulation states that sponsors must maintain a system to manage quality throughout the trial process and archive TMFs for 25 years after studies end. Regardless of their outsourcing model, European sponsors have ultimate regulatory responsibility for the trial master file (TMF), including study archives.
For emerging biopharmas, this might seem like a daunting task. However, these sponsors can implement a “divided eTMF” model. In a divided model, sponsors adopt an eTMF but continue to outsource the majority of TMF management to CROs, increasing compliance while conserving resources. That way, they can take the first step toward transforming their clinical trial oversight and establish the foundation for long-term success. Once their clinical operations volume increases, they can evaluate whether they want CROs to work in their sponsor-owned eTMF to increase oversight and control.
In this blog, I will share how to use Vault eTMF to increase compliance throughout study conduct, closeout, and archival while outsourcing in this “divided eTMF” model.
Step 1: Manage sponsor documents during the study
You can use Vault eTMF to manage sponsor documents that you wouldn’t manage through your CROs. This includes documents like risk management plans, oversight plans, and internal SOPs.
That way, you continue to outsource the majority of TMF management to CRO partners but ensure compliance of sponsor documents in a validated system, rather than storing them in SharePoint or another repository.
For the head of clinical operations at a growing European biotech, the necessity for sponsor-owned eTMF is clear: “We know that a sponsor must have a TMF. It is absolutely appropriate to delegate most of the management to CROs, but there will generally be a set of documents that need to be managed by the sponsor. You need to show auditors where all the documents are held — by both sponsor and CRO.”
Read more about how Vault eTMF supports sponsor compliance.
Step 2: Transfer documentation from CRO partners at study closeout
At the end of the study, you can then migrate study documents and data from your CRO partners into Vault eTMF. Leveraging TMF Transfer, a built-in feature, eliminates manual end-of-study migrations and enables seamless transfers between sponsor and CRO Vaults. You can also repeat the transfer process with CRO partners without additional setup or cost.
For one emerging biopharma, TMF Transfer reduced TMF migration preparation time by 84% — from 25 to 4 days. The biopharma’s head of TMF says, “Everything is automatically in the system in a matter of minutes. TMF Transfer takes exponentially less time.”
TMF Transfer includes:
- All country and site records, including archived records
- Approved documents, including the source document and rendition, approved versions, audit trails, standard document fields, and attachments
- Other document and data records needed to meet regulatory requirements
Step 3: Drive compliant study archives
Once you have the complete TMF in your system, you can initiate a single-click archival process. The system includes built-in prerequisites to ensure archival readiness.
Once a study is archived, you control who can access archived data and documents. You can also search, filter, and report on archived content. The designated archivist role can upload documents to the eTMF archive to ensure a compliant archive management process, and the system maintains the same structure as your active TMF to improve inspector navigation.
The TMF director at one biopharma explains why compliant archival is critical: “Archival is as important as trial operations because, when the study is over, all you have are these documents to show how the study worked.”
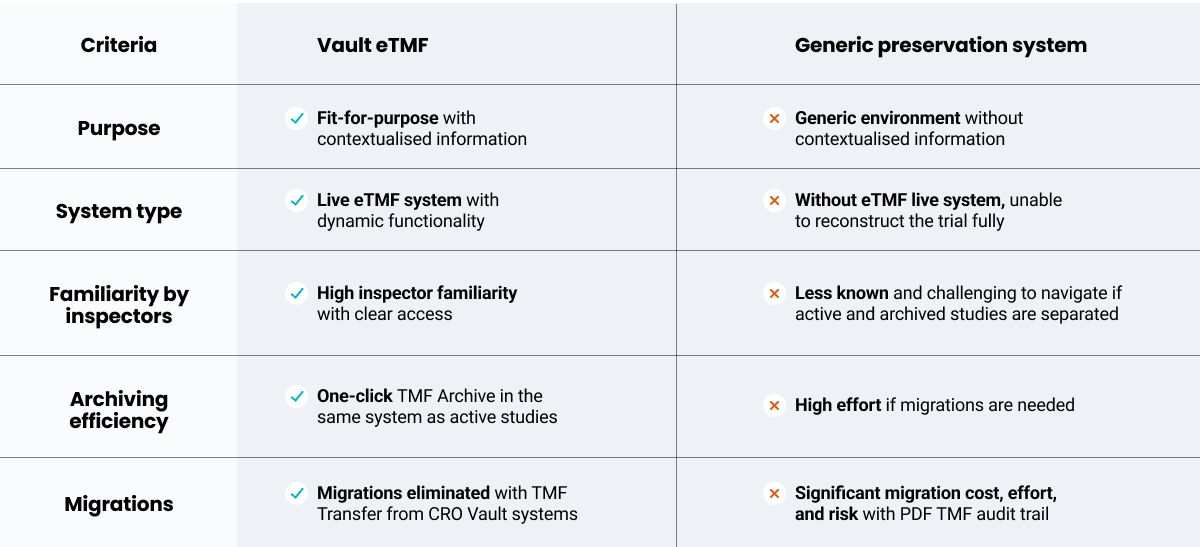
TMF Archive also conforms with the EMA’s guidance on computerized systems, which requires dynamic data files including audit trails. When comparing TMF Archive to generic preservation systems, you can see how a fit-for-purpose solution increases efficiencies and compliance:
Learn more about how to improve trial efficiency and inspection readiness with Vault eTMF.
