A Growing Dermatology Biopharma Improves Oversight with In-House Drug Safety
Brought safety in-house to improve oversight while continuing to outsource PV activities
Cut PADER reporting time from 30 days down to just two weeks – a 50% improvement
Set up its safety database in just a few months to prepare for an upcoming PMDA approval
Many smaller biotechs with limited resources rely heavily on outsourcing their pharmacovigilance (PV) functions to contract research organizations (CROs). But, “outsourcing models only work when you have proper oversight,” says the PV director at a dermatology biopharma that launched its first commercial product in 2022.
Smaller companies may see sacrificing data ownership and real-time oversight as the price to pay for outsourcing safety activities. However, the PV director, with over 10 years of drug safety experience, believes even the leanest PV teams can improve oversight by bringing safety in-house. “I’m a big advocate for owning your data. The lift is not any different when you own your system. You’re still paying the same amount of money and it takes the same amount of work. But, if you’re paying someone else to do it for you, you don’t get visibility into it. It also doesn’t look good to an auditor when you have to go to a vendor to get your data,” she explains.
“I’m a big advocate for owning your data. The lift is not any different when you own your system. You’re still paying the same amount of money and it takes the same amount of work. But, if you’re paying someone else to do it for you, you don’t get visibility into it.” – Pharmacovigilance director at a dermatology biopharma
The company’s three-person PV team brought safety in-house while maintaining full control over its data and operational oversight. “With Veeva Safety, I don’t have to wait for someone to pull my data for me. I can see my data in real- time. I know how it’s flowing and how long it takes to process a case,” says the PV director. “If an agency walks in and wants to see a report, I can run it for them right away. Without that oversight, you can’t give an inspector the timely answers they’re looking for.”
Outsourcing without sacrificing data oversight
With Veeva Safety, the company’s PV team manages safety in-house and gives vendors access to their system. Currently, they outsource case intake to a CRO. Then, a service provider uploads case information or adverse events into Vault Safety to process those cases.
Once their service provider has processed the cases, the PV team spends the majority of their time on data reconciliation and follow-up activities. This helps ensure their data is accurate and complete. “We review all the information from our call center. We also check to make sure all the source documents are correct before they go over to our processing vendor,” says the PV director. “From an audit perspective, everyone needs to be on the same page at all times. For example, if someone says they sent me five cases, I want to be sure that I got five cases and both parties signed off on them.”
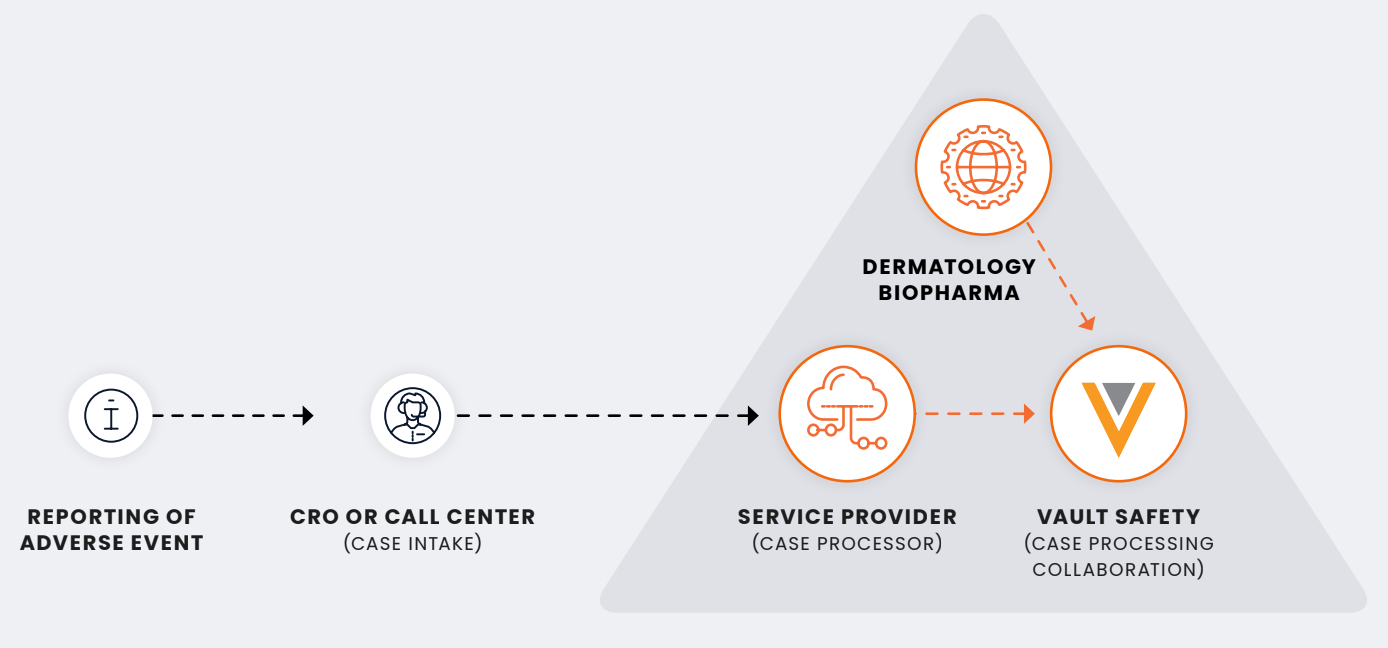
Safety data ownership for real-time visibility and control
Some sponsors may not realize that outsourcing safety activities will impact their data access, visibility, and ownership. “Most PV teams think ‘Of course we own our data. Our partner just stores it for us,’” says the PV director. “But, if you have to pay a fee to access or get it, then you don’t own your data.”
Typically, most small PV teams rely on their vendors to access their safety data. When a company reaches out to its vendor with a request, it is up to the vendor and the contract when they respond. Even for the most urgent requests, it can take anywhere from several hours to multiple days to get the data and may come at a financial cost.
One of the Veeva Safety features that the company values most is the ability to quickly run reports that are based on real-time data and stored in one place. The company’s PV director most frequently uses a report that reconciles serious adverse event data from its clinical team on a monthly basis. The PV team has also created custom reports to track key metrics. For example, they have a report that tracks case volume and changed components to ensure their vendor is billing them correctly. They also have a report that checks the PADER for errors before submitting it to the gateway. “I pull this report at least once a week to make sure there are no errors in the coding. And, if there are, we know exactly what to send back to our vendor,” says the PV director.
Before implementing Veeva Safety, the PV team would take the full 30 days allotted to them from data lock to submit their PADERs to the FDA. Since streamlining their reconciliation reporting with Vault Safety and collaborating with Vault Submissions Publishing, their reporting time is down to just 2 weeks – a 50% improvement. In the future, the company’s goal is to further shorten its reporting time to 7-10 days using auto-submissions.
Defining KPIs and success metrics
Veeva Safety helps the biopharma ensure that vendors process cases within their contractual agreed-upon timeline. “Meeting those time frames is important for oversight,” says the company’s PV director. “You don’t ever want to be in a position where you miss a serious case and your vendor is late to process it.” She also appreciates having visibility into the company’s safety data. “It’s important to be able to see your data flowing in real time to know if there are any potential safety signals.”
The PV team also tracks the difference in case dates. “It’s a simplistic metric, but it’s important to make sure that you’re on top of things,” says the PV director. In Veeva Safety, she filters through her workflows, then filters by date to find cases that have been sitting in data entry or QC without being processed. “Once we find those cases, we reach out to our vendors to ask why they haven’t been processed or if there’s a problem with them.”
Tips for audit-readiness
The PV director recommends mock-audits – especially for first-time audits. Her team developed a checklist for audit readiness, including:
- Regulations: Take a look at what evidence regulators are expecting to see. Do you have a folder with the information to answer each of their line items?
- Contracts: Do you have all your contracts? Are they signed and dated? Are they easily accessible?
- Reporting: Do you have a quick or pre-generated report that you can run if an auditor wants to see all the serious cases you’ve submitted? Can you pull that within 15 minutes?
- SOPs: Have you prepared your external vendors to share their SOPs if necessary?
Above all, having a single global repository to house the biopharma’s data and information gives the PV director confidence during an audit. “You never want to leave an auditor hanging around waiting for a response or leave your team scrambling looking for documents,” she explains.
How to seamlessly scale globally
Now that the PV team has created a strong foundation of operational oversight, they are scaling their safety processes globally. The company has partnered with a Japanese pharmaceutical company to file for approval of their latest treatment with the PMDA. As the company prepares for its upcoming approval, the PV team is planning to use Veeva Safety to communicate with their Japanese counterparts while ensuring they meet both FDA and PMDA regulations. “As your company grows, you have more countries and responsibilities. It’s great to be able to scale your database quickly,” says the company’s PV director. “Complex updates like these usually take a year or two. But, with Veeva Safety, we can make them in a few months.”
“As your company grows, you have more countries and responsibilities. It’s great to be able to scale your database quickly. Complex updates like these usually take a year or two. But, with Vault Safety, we can make them in a few months.” – Pharmacovigilance director at a dermatology biopharma
Embracing new safety technology
For emerging biopharmas, bringing a safety system in-house might feel daunting. There is a common misconception that sponsors who own their data will no longer have any vendor support. In past roles, the PV director felt like she was left on her own after implementing a new PV system. But, that has not been her experience with Veeva Safety. “We have a strong support team with Veeva Safety. Even after going live, we’re still meeting with managed services regularly and getting real-time responses to our questions,” she says. “In all my years, I have not had this kind of support from a vendor.”
Although PV professionals are typically risk-averse, the company’s PV director recommends being open to trying new tools. “Don’t be scared of technology. It doesn’t hurt to see what resources are available for you,” she advises. “Even if you’re not ready right now, what if you’re ready in a year or two?”
Learn more about how bringing PV in-house drives better oversight and faster data analysis.




