Blog
Bringing Clinical Data Together – Improving Patient-Centricity in Clinical Trials
Jul 28, 2017 | Veeva
Jul 28, 2017 | Veeva
Managing Director, Deloitte Life Sciences
The goal of the life sciences industry is to improve the health of patients. A diverse group has to come together including biopharma, medical device companies, CROs, investigator sites, payers, providers as well as regulators to deliver care that is changing the way we collect clinical trial data. Focused on improving data quality, we see patient activism, adoption of use of electronic health records, and data from wearables resulting in opportunities to improved patient-centricity. These trends have also led to an increase in volume and variety of data in clinical trials which means data is no longer coming from EDC alone but needs to be integrated from multiple sources including case report forms to electronic health records, wearables, social media data and biomarkers.
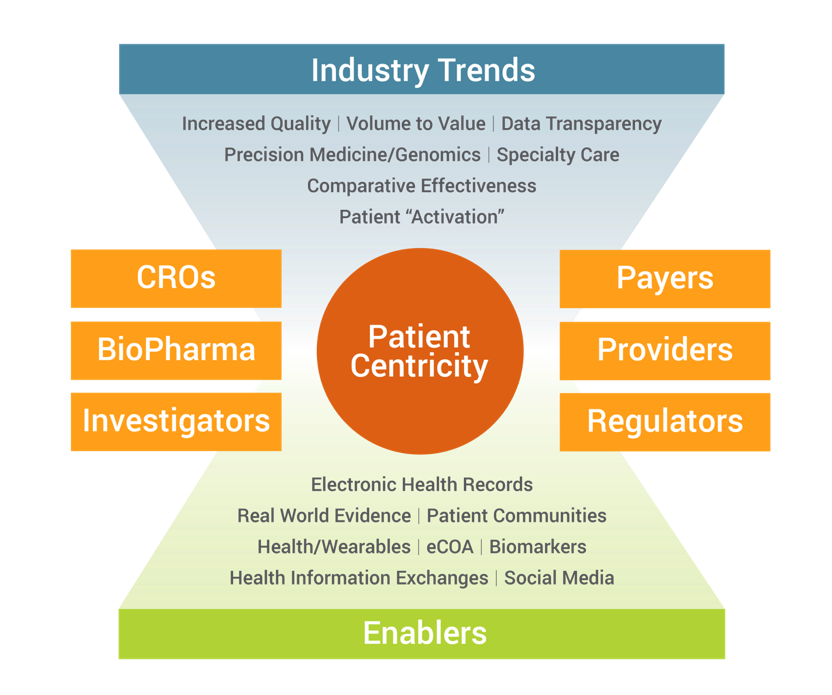
Figure: Increased Focus on Patients in BioPharma Development
We now have more data points than ever before resulting in the drastic increase of the complexity of trials. There are millions of data points in a day for a phase III clinical trial that needs to come together in a meaningful way to deliver actionable insights. To transform clinical trials and improve patient-centricity, clearly the industry needs to look at clinical data differently than we have in the past.
The industry has made a giant leap in moving from error prone paper-based case report forms to electronic data capture (EDC) systems in the past two decades. But EDC systems evolved in a way that made them look like electronic version of case report forms. The advent of digital CRFs within EDC systems allows us to search and find what you need with a lot more ease than paper but not all the data needed for modern clinical trials reside in a case report form.
While EDC systems have been a giant leap ahead for clinical trials, we still struggle with their inability to integrate all the varied data sources and handle the volume of data that is available today, and to glean actionable insights from these massive volumes of data. All these changes raise an important challenge that needs to be addressed on the issue of data governance and standards. The questions to ask – just as we did when we moved as an industry from paper based CRFs to EDC Systems, is where are we headed next from traditional EDC systems and what does the future look like?
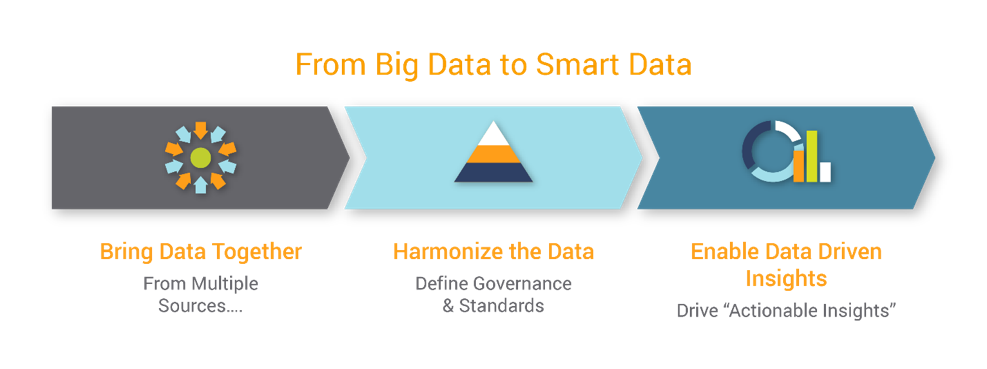
Figure: Focus is on the Data
The Future of Clinical Trials: Focus is on the Data
The future of clinical trials is dependent on modern infrastructure that replaces traditional EDC systems and brings together a large volume of data together from varied sources. This modern EDC system would need to harmonize data from all the various sources and deliver actionable insights. The modern EDC system needs to tie data back to key performance indicators and drive key risk indicators. It needs to share this data seamlessly across all team members irrespective of their functional area and allow data visualization that is personalized.
The good news is the industry is ready for innovation and EDC technology vendors will need to deliver on this vision for the future with a modern EDC that leverages big data and make clinical trials smarter and mitigates risk early, drives efficiency, ensures data quality, and delivers regulatory compliance- all while be cost effective.
A Veeva Perspective
Total data management is the key to a better EDC. The current EDC systems will become quickly redundant as they fail to meet the ever increasing need for speed, regulatory compliance, and cost around the management of this increased volume and variety of data. Building a modern EDC requires us to start from scratch guided by patients, sites, sponsors, CROs, and users.
Built on a modern unified clinical platform, Veeva Vault EDC is delivering on this vision for a modern cloud-first EDC platform. Vault EDC allows you to run the trial you want, not the one your technology limits you to. You can quickly deploy studies in weeks and not months, and make in-flight amendments without migrations or downtime. The ability to integrate and maintain complete and concurrent data, including non-CRF data, in a trial provides real-time insight and dramatically improves productivity. To learn more on how Veeva is delivering on this vision, listen to this podcast by Richard Young, VP of Vault EDC.
