Blog
Centralized vs. Decentralized RIM Operations: Q&A with Jazz Pharmaceuticals on Regulatory Data Management
Jun 17, 2024 | Paul Attridge
Jun 17, 2024 | Paul Attridge
As the phased implementation of IDMP continues, data integrity is now a business – as well as a regulatory – priority. A key enabler is having the right people and data models in place to navigate ongoing changes efficiently.
During a recent conversation with Paul Attridge (vice president of Vault RIM in Europe), Gareth Markham, associate director regulatory information management at Jazz Pharmaceuticals, shared team structure, speed, and scalability issues stemming from IDMP’s phased implementation. He also discussed how Jazz optimized its regulatory processes and whether centralized or decentralized data models are more effective for managing regulatory submissions in the context of the following three key challenges:
- Balancing expertise and efficiency in regulatory data management
- Embracing a data-centric, growth mindset for operational agility
- Mitigating risk and increasing cost efficiency
Challenge #1: Balancing expertise and efficiency in regulatory data management
It can be difficult to optimize for speed and accuracy when managing regulatory data. Remote teams range from dozens to hundreds of employees, resulting in time-consuming data hand-offs, while a lack of understanding of the data can lead to costly errors and hours spent re-reviewing records.
Paul Attridge (PA): How do you identify who should own each part of the data entry process?
Gareth Markham (GM): Understand the strengths of your data management teams and weigh the downstream consequences of delays or incorrect data. At Jazz, centralized teams know the systems and system requirements, so [they] understand how to manage regulatory submissions. Decentralized teams know the data and can ensure accuracy.
We also rely on experts to maintain record completeness, and reduce time-consuming oversight on the backend.
Challenge #2: Embracing a data-centric, growth mindset for operational agility
As IDMP standards are phased in, it can be challenging for global companies to scale changes across their global footprint.
PA: How did you prepare to react quickly to regulatory changes?
GM: Health authorities are becoming more data-centric. While IDMP is driving this in the near term, further regulatory requirements are expected globally, and the industry needs to prepare. At Jazz, we needed to reframe how we think about regulatory submissions. Regulatory is no longer just about documents, it’s data as well. This meant changing our perspective and embracing a ‘growth mindset’ so we could respond quickly. By consolidating responsibility for regulatory submissions to a specialized team, we were able to react to changes quickly and efficiently.
Challenge #3: Mitigating risk and increasing cost efficiency
With the right systems in place, companies can quickly complete accurate submissions, while managing costs and mitigating risks.
PA: There are of course financial implications to completing regulatory submissions more quickly. How do you optimize cost efficiency while ensuring alignment with industry requirements?
GM: It’s important to analyze cost effectiveness through the lens of a changing regulatory process and consider how it impacts productivity and your systems. At Jazz, we took the perspective that a decentralized approach is more scalable for us as we enter new markets.
We also knew that if records were created inaccurately, that would consume a lot of resources. Decentralized teams know the data intimately and can mitigate the risk of inaccuracy; while centralized teams are responsible for ensuring up-to-date information is stored properly and reducing the number of hand-offs, which is critical when it comes to audits or health authority (HA) requests for information.
PA: One final question. From Jazz’s perspective, what is the most effective data management model?
GM: It’s essential to get roles and responsibilities right in regulatory operations to be responsive to changes while operating with speed. It reduces risk, promotes accountability and ownership, and enables us to do more with less. There isn’t a perfect one-size-fits-all solution. Regulations continue to evolve as time goes on, so your model has to adjust to accommodate.
For example, a decentralized model might be the best approach when investing in future skills as changing the mindsets of individuals is easier than fundamentally changing the way an organization operates. Training and technical proficiency work best with a more centralized approach as consolidated training is easier to roll out and manage.
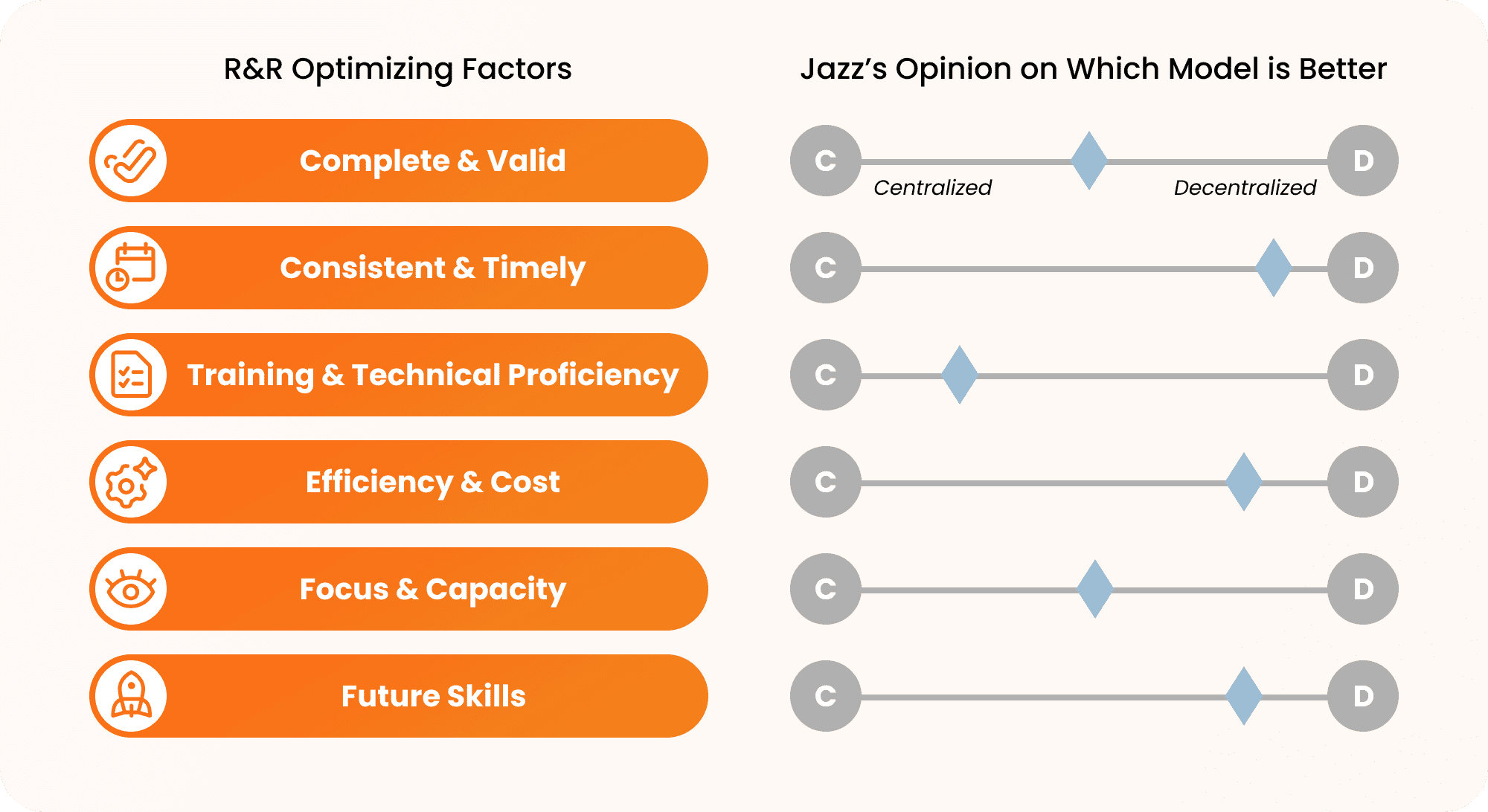
Additionally, centralized or decentralized isn’t a static point on a graph. It’s a fluid and sliding scale, depending on what part of the journey you’re on.
To learn more, visit our IDMP Resource Hub or watch our recent webinar with Jazz Pharmaceuticals.
