Blog
MHRA Identifies Common Flaw in Managing Protocol Deviations
Jan 06, 2022 | Paul MacDonald
Jan 06, 2022 | Paul MacDonald
Understanding protocol deviations is critical to preserving the scientific integrity of your study – so why are many companies doing it wrong?
In one audit of protocol deviations, 304 of the 447 deviations reported to an ethics committee were related to study procedures such as missing visit windows, missing assessments, and incomplete case report forms.1 If this audit is representative, then the majority of reported deviations are subject-related protocol deviations that will surface within the EDC. Given the scale and importance of deviations in a trial, we need to stop and take notice of the MHRA’s recent comment that many companies are managing them incorrectly.
The traditional approach
Many traditional EDC systems track deviations at the datapoint level with limited descriptive information and no workflow capabilities. As a workaround, study teams often create a dedicated eCRF for monitors and study teams to track and assess protocol deviations. However the UK regulator, MHRA, frowns on this practice, stating:
We have seen the addition of protocol deviation forms into the eCRF for monitors to enter the information and the study management team to assess and document their review. This is not acceptable as this is an entry into the investigator’s eCRF (for example, form page).2
They go on to differentiate between data captured within an eCRF and data captured elsewhere within an EDC system:
It should be noted that workflow systems within the eCRF software itself, such as coding or queries, which allow sponsor staff to enter information, are acceptable as these are not trial specific data entry screens (which are CRF forms)
One option they put forth is to create a wholly separate database for managing deviations. Whilst this creates a clear separation between sponsor and investigator data, it also breaks the connection between the two. Maintaining separate systems requires duplicate data entry and therefore additional checking and manual reconciliation.
Tracking protocol deviations within the EDC as independent objects that are separate from–but related to–the eCRFs provides the optimal balance.
A Modern Data Management Approach
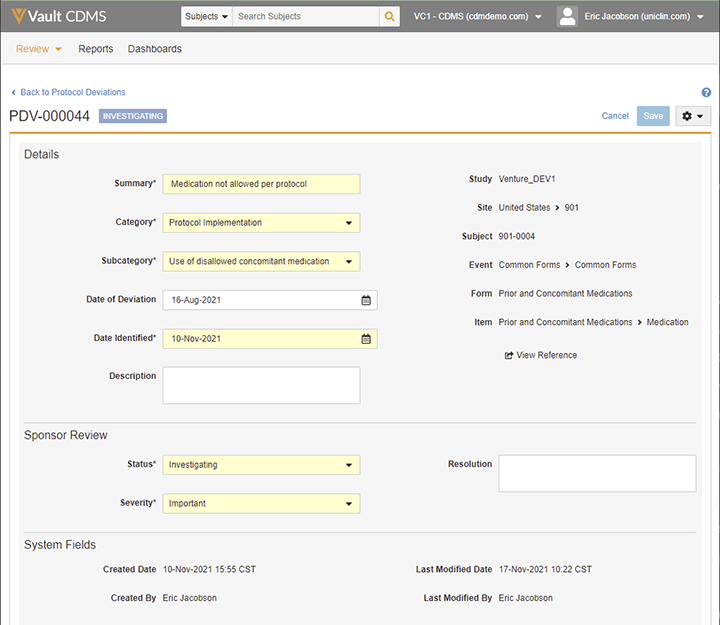
A modern EDC should provide a module for handling PDs that achieves the regulatory requirement of separating sponsor and investigator data, as well as the business goals of efficient timely reviews and study insights.
Protocol deviations in Vault EDC are managed as a separate object, in their own database table distinct from the CRFs. Comprehensive information is captured so sponsors get the information they need to identify problems when they arise.
Veeva enables you to classify, categorize, and associate PDs along three different dimensions. Tracking PDs with additional and more granular metadata provides your study team with valuable information that can help identify systemic problems. Three PDs at a site may not signal a problem, but three PDs at a site surrounding subject eligibility would certainly raise cause for concern.
TransCelerate’s process guide for protocol deviations defines classification and categorization as follows:3
- Classify is defined as determining if the protocol deviation is important or non-important.
- Categorize is defined as the type of protocol deviation (e.g., inclusion/exclusion).
We recommend a third level of organization, which we will term “contextual association” and define as:
- Contextual association is defined as specifying the context or level at which the deviation was made (e.g., subject-level, visit-level, or form-level).
Designating the context of the deviation, e.g. is this a visit-level deviation or a form-level deviation, helps organizations scope the data impacted by the deviation, which supports downstream data management and programming tasks. For example, if a visit was missed, all the data pertaining to that visit would be impacted. Sponsors shouldn’t need to add a question “Did this visit occur?” as a work-around for tracking visit-level deviations. Instead, they should designate that the deviation was on the visit, and by association, all constituent fields would be impacted.
Tracking the context also provides additional study insights. For example, if a PD is created at the form level, such as an informed consent form, it stays associated with that form. This feeds valuable information back to the study team, as they can look across all their forms and see which are generating the most deviations.
Categorization ideally captures category and sub-category designations to better support internal analyses with additional descriptive information. For example, in the category Protocol Implementation, sub-categories may be: Missed visit, Missed assessment, Participant received wrong treatment, or Participant seen outside of visit window. Offering sub-category options provides additional detail to feed analyses for trends or evaluations of the underlying reasons or root cause of a deviation.
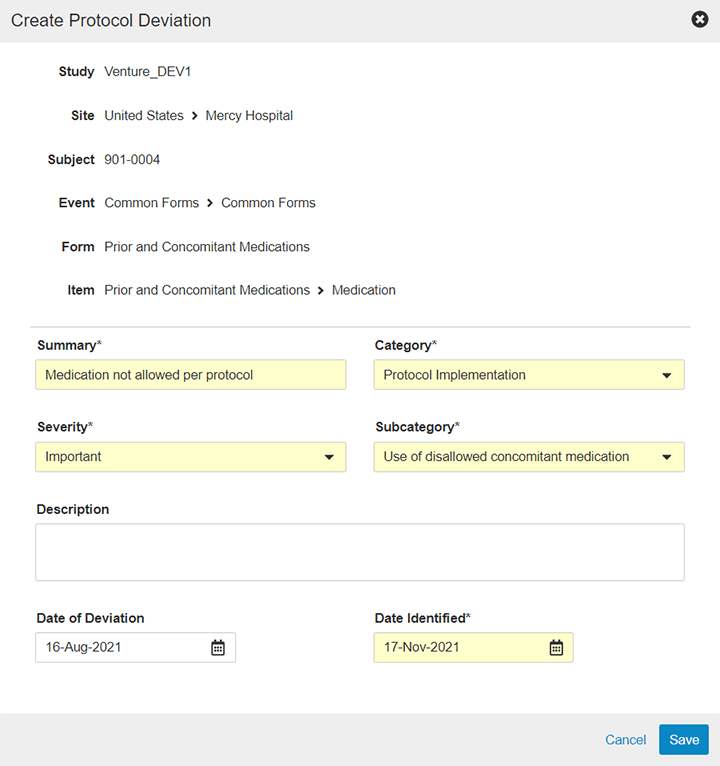
Classification defines the deviation’s severity. Vault EDC’s severity or importance ratings are configurable to allow each sponsor or CRO to use terminology they are accustomed to or to reflect industry best practices. All of these fields are visible within a filterable deviations view, as well as within the native dashboards and reports.
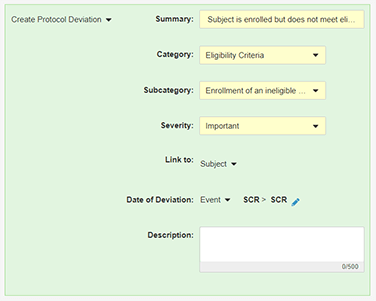
Veeva’s rules engine can assist with creating and classifying deviations. If you want to have Vault automatically create a protocol deviation when certain conditions are met, create a Create Protocol Deviation rule to do so. Protocol deviations created by rules are “programmatic,” while those created by monitoring users are “manual.”
Management Tools
The first step in TransCelerate’s PD decision tree4 is assessing whether the event or discrepancy is a true protocol deviation and then assessing its importance. Over-reporting PDs increases the “noise” in the system and can overshadow information that is important to patient safety. Whereas under-reporting PDs threatens the integrity of the trial and may jeopardize patient safety.
Veeva’s EDC provides comprehensive management tools for subject-related deviations. Workflows help sponsors manage deviations and track their status, severity, and resolution. Again, all information about the deviation is captured within the EDC, but not within the CRF, maintaining a clear distinction between investigator data and sponsor-entered data.
Conclusion
Veeva is bringing innovation to the process of managing PDs in three important ways:
- Remedying EDC deficiencies by storing PD information within the EDC, associated with the data in question and separate from the eCRFs.
- Providing in-depth classification capabilities that will help study teams surface problems quickly and provide appropriate mitigation strategies.
- Providing a dedicated interface to assess the deviations and track resolutions.
A future blog on protocol deviations will cover the Vault CDMS to Vault Clinical Connection, and how transferring subject-level deviations into Vault CTMS provides a holistic view of deviations across your study. Having a unified and connected platform that offers best of breed capabilities reduces risk, while improving efficiency and quality. Watch the demo and learn more about how the Vault platform provides an end-to-end solution for protocol deviations.
Additionally, to learn how your team can reduce protocol amendment cycle times by 70%, register for this upcoming webinar with Bioforum.
