Building the Business Case
for Sponsor-Owned eTMF
100
studies across R&D and medical affairs
1000+
internal and external users
Moved from CRO to
sponsor-owned eTMF
Now an advocate for sponsor-owned eTMF, a global biopharma shares why changing course improved TMF inspection readiness and tips for building the business case.
Every business case will have to answer the same question: will the return on investment (ROI) be positive? Yet for sponsors seeking their own eTMF system, focusing solely on a cost-benefit analysis has limitations. The risks of not changing course can be a compelling part of the argument. And unless a team can advocate for a system once it is in place, it is unlikely to reach its full potential within the organization.
As it began its partnership with Veeva, one global biopharma was running nearly 100 studies in R&D and across global/local medical affairs. Its eTMF landscape was complex, encompassing 1,120 internal and external users, and spanning 10 departments and 20 contract research organization (CRO) partnerships. Two recent acquisitions added new SOPs and processes to account for.
Although it went live with Veeva eTMF in November 2015, the biopharma’s first implementation offered users the choice of either the company’s or a CRO’s eTMF. Giving users discretion over which system to use led to several challenges during eTMF retrieval, triggering a transition to full ownership in July 2018.
In 2022, the system management team built a successful business case to secure finances, resources, and senior leadership buy-in for a fully standardized eTMF. It deployed an upgraded eTMF and new processes and SOPs in November 2023.
Using its own eTMF has helped the biopharma achieve its principal objective of inspection readiness. As the company’s R&D director of systems, standards, and services, observes: “The main benefits of this system are to be inspection ready at any time and generate metrics and key performance indicators (KPIs) so we can proactively identify deficiencies.”

Structuring your business case
A successful business case doesn’t just list the problems facing the organization today but is convincing and specific about how the investment will address them. It should also outline the risks of leaving pain points unaddressed.
For sponsors, using a CRO’s eTMF system could lead to inefficient quality control (QC) and oversight, multiple trackers, or challenges storing confidential documents. Additionally, the biopharma found it couldn’t use the latest Veeva features as some of its providers relied on older configurations. All of this resulted in a lack of confidence in inspection readiness.
Another common challenge is that each CRO partner may follow non-standardized processes and SOPs, leading to confusion between different eTMF versions, re-training, or duplicated repositories. This heightens the risk that CROs mistakenly use obsolete or inappropriate document versions.
Your business case should be upfront about the challenges you’re encountering and then link each one to the intended outcomes (for example, how owning your eTMF could improve inspection readiness)
[Figure 1].
The most compelling proposal will always be aligned to the overall company vision, so explain how a sponsor-owned eTMF aligns with your company goals. For the biopharma, owning its eTMF removed the risk of non-compliance by introducing a single source of truth for data. Harmonized eTMF management then became possible across all studies, as did new eTMF quality controls. Users soon benefited from following common rules in a single procedure rather than working in a confusing array of eTMF systems, while document owners gained visibility of trial completeness, including missing, duplicated, or expired documents.
Selling your vision
The executive summary of a business case is often a showcase for positive ROI for senior leadership.
However, a cost-based business case can be difficult to prove: licenses are direct, whereas the exact eTMF cost when working with a CRO is unknown. To tackle this challenge, one option is to compile a tracker comparing available solutions and explain the impact of the project not proceeding [Figure 2].
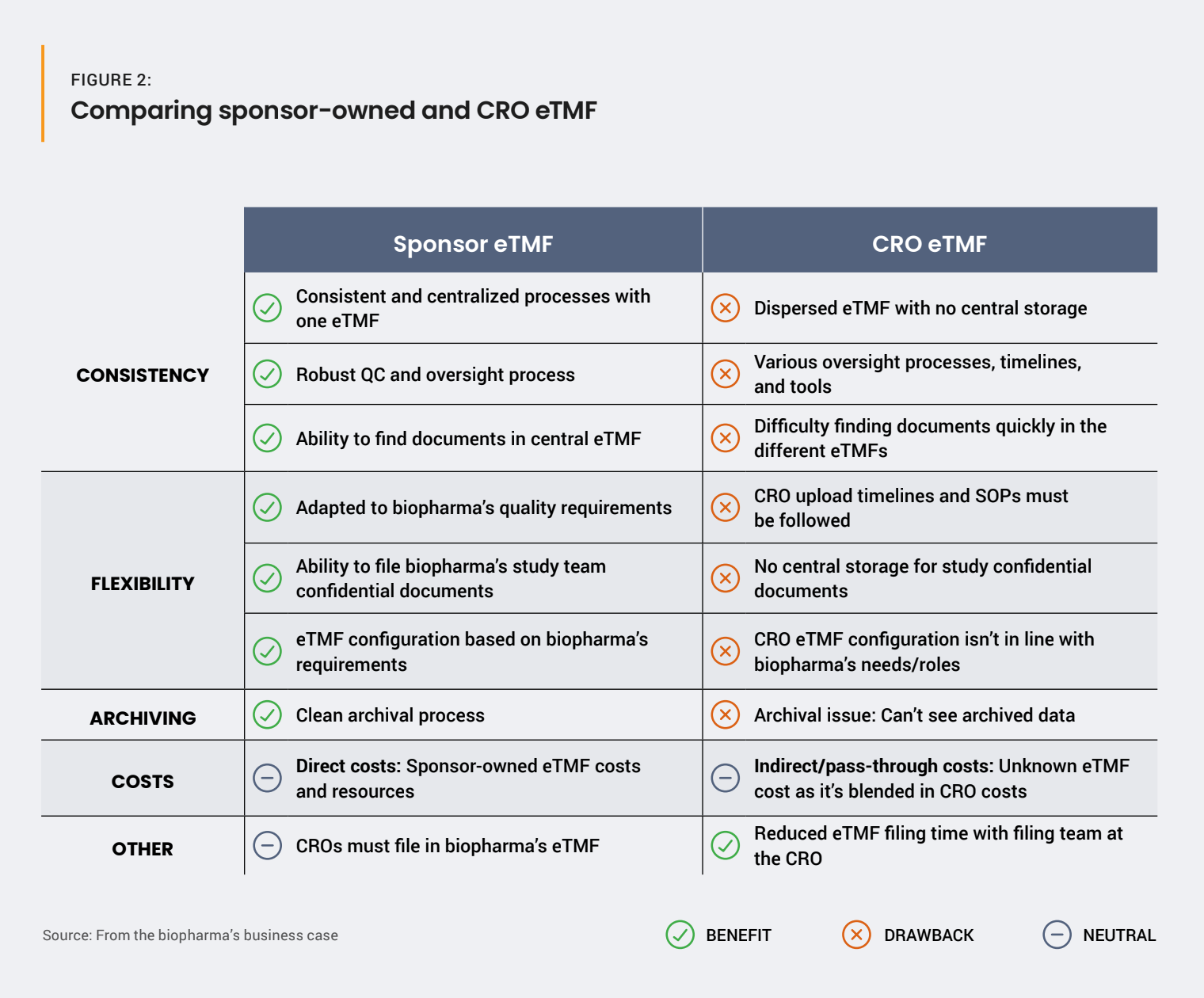

This could help your organization conclude that a sponsor-owned eTMF facilitates external partner oversight, confidential document management, and complete system validation. This must be weighed against the extra resources needed for training, implementation, and maintenance, as well as direct costs [Figure 3].
Advocate for your vision
Sponsors are seeing the benefits of owning their eTMF, such as increased control and oversight, as well as simplified workflows.
It helps if the system becomes more visible to the wider company. “Never miss an opportunity to sell your project,” advises the R&D director. “You could tell your story at events and share achievements on company channels.”
Successful teams do this early and often by:
- Aligning their plan to company initiatives
- Emphasizing risk avoidance
- Communicating broadly, from leaders to users
At times, you may need to defend your chosen system as well as advocate for it. Laying the groundwork early and building a robust business case will help others recognize the value and vision you have in mind.
Learn why sponsors are switching to Veeva eTMF.




