KCR: Three Lessons from Our Journey to Modernize and Unify Clinical Trial Operations
KCR is a medium-sized CRO with a global reach. They use the Veeva Clinical Platform to drive their digital transformation. The team has managed dozens of studies and hundreds of thousands of documents, all while dramatically improving TMF quality measures.
To support a unified approach, KCR implemented Veeva eTMF in 2014, followed by Veeva CTMS in 2019 and Veeva EDC in 2021. Magdalena Matusiak, KCR’s head of data science and documentation services, shares three lessons for success.
Before implementing new technology, understand the capabilities and use those learnings to develop new processes to maximize value.
Before implementing Veeva CTMS, the KCR team learned the full range of capabilities within the application. After that, Matusiak said, “We thought about how to change our processes, approaches, and SOPs so we could really benefit from the technology.”
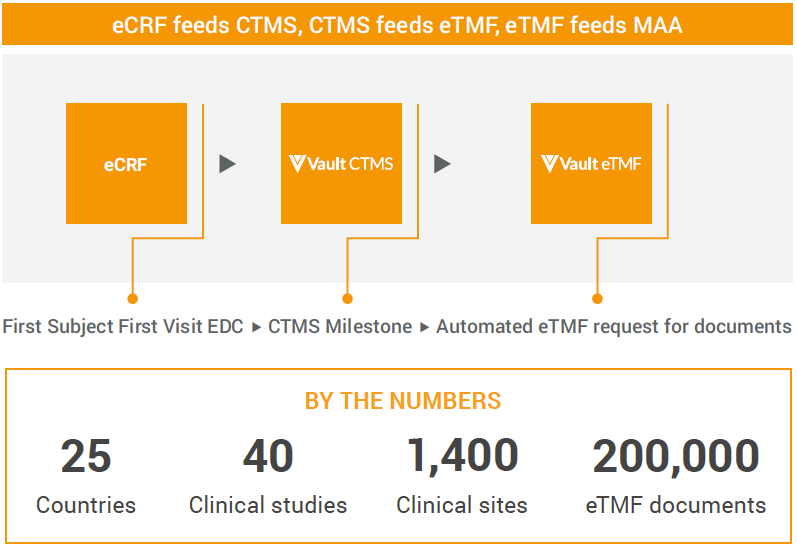
Veeva CTMS became the team’s communication and process management tool through which subject data and trial documentation flow. They also established automated workflows so that actions trigger downstream activities throughout the Veeva Clinical Platform, rather than relying on email and other external channels for study communication.
For example, a case report form that marks a study’s first subject first visit in Veeva EDC is automatically visible in Veeva CTMS. Once the milestone is complete, the system generates an automated request in Veeva eTMF for milestone-associated documentation.
Define clear responsibilities for each role, and empower subject matter experts to take ownership of their documentation.
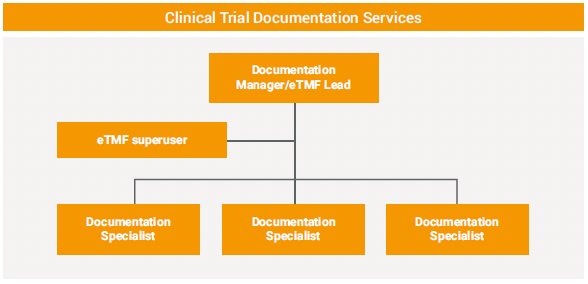
KCR established new teams with clear roles, as well as guidelines for processes such as data entry and quality control (QC). For example, clinical trial assistants were previously responsible for all documentation, but KCR created a new clinical trial documentation team with responsibilities divided among subject matter experts.
“Defining clear roles and responsibilities is the key to success when we think about implementation of new technology,” Matusiak said.
Use technology to increase transparency and aggregate data to measure success.
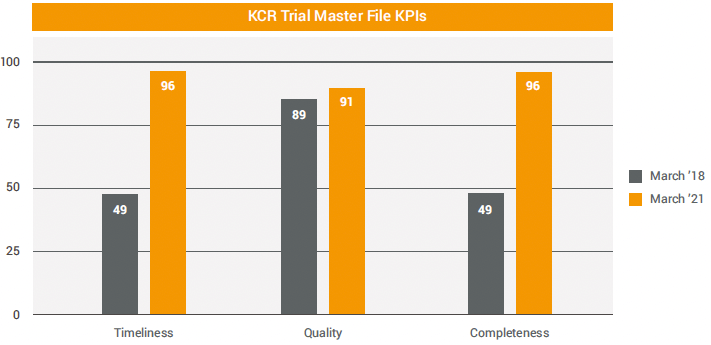
The clinical trial documentation team helped define and apply KPIs to support the technology implementation. They also aggregated data from their Veeva applications to drive improvements such as increased TMF health. In three years, the team nearly doubled timeliness and completeness of eTMF documents and maintained high quality.
“Even the best technology is useless if we don’t have data in sight,” Matusiak said. “Data helps to ensure success during technology implementation and beyond.”
Learn more about standardizing clinical trials for efficient studies.





