Veeva Disclosures
Centralize and Streamline Clinical Trial Disclosures
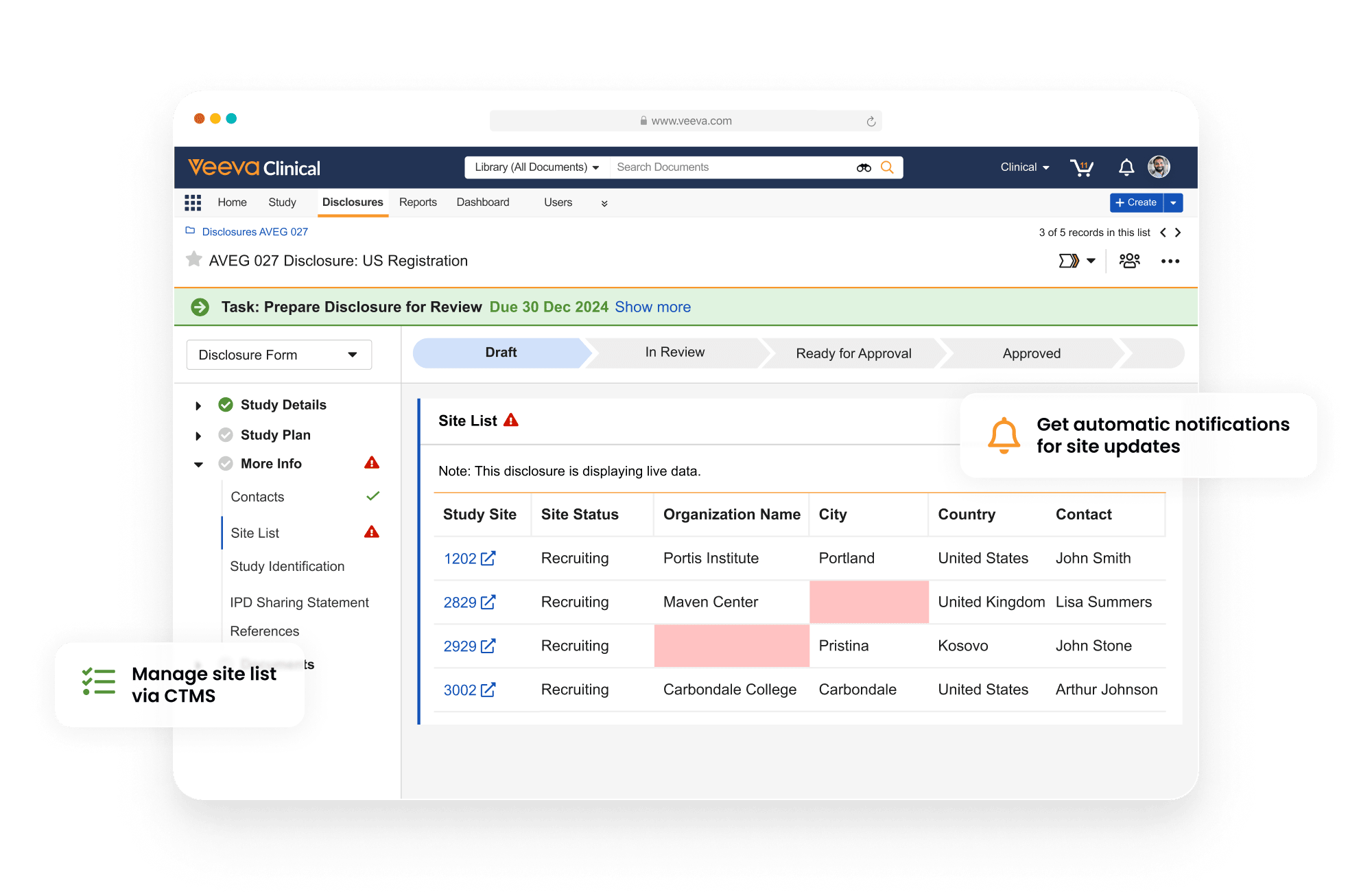
Disclosures manages the sharing of study registrations and results disclosures with registries. It supports the entire process, including capture, review, workflow, approval, reporting, and XML generation and submission.
Pre-configured registry rules make it easy for users to comply with regulatory requirements. Automated alerts for changes in study data and milestones keep users informed about important tasks and prompt them to take action. Automatic filing of disclosure documents in eTMF helps generate proof of submission. Reports and dashboards provide visibility into global submission status, operational progress, and key metrics.
Disclosures uses data from eTMF, CTMS, and Study Startup, eliminating third-party integrations and manual entry.
Announced 2023 Status Early Customers 1-10
See how unified systems simplify disclosure management
Overview
Centralize and Streamline Clinical Trial Disclosures
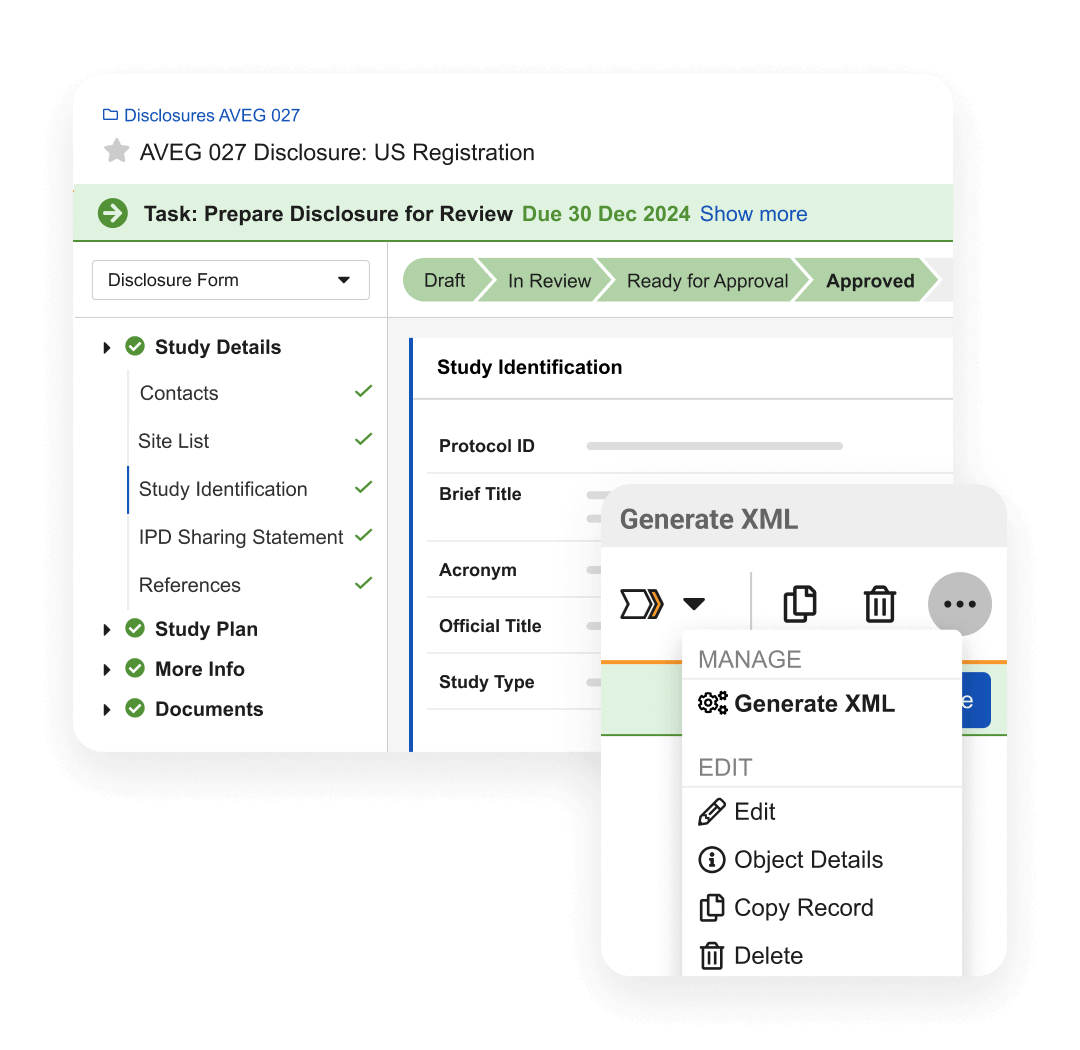
Disclosures manages the sharing of study registrations and results disclosures with registries. It supports the entire process, including capture, review, workflow, approval, reporting, and XML generation and submission.
Pre-configured registry rules make it easy for users to comply with regulatory requirements. Automated alerts for changes in study data and milestones keep users informed about important tasks and prompt them to take action. Automatic filing of disclosure documents in eTMF helps generate proof of submission. Reports and dashboards provide visibility into global submission status, operational progress, and key metrics.
Disclosures uses data from eTMF, CTMS, and Study Startup, eliminating third-party integrations and manual entry.
Impact
Accelerate compliant and accurate
disclosure submissions
~80 %
Registration fields automatically pre-populated
~80 %
More accurate
registry
submissions
~60 %
Faster trial
registration
set-up




