Veeva eCOA
Deliver eCOA at Scale
eCOA (electronic Clinical Outcome Assessments) captures questionnaire responses directly from clinical trial patients (ePRO), clinicians (eClinRO) or patient caregivers (eObsRO) using an app or webpage.
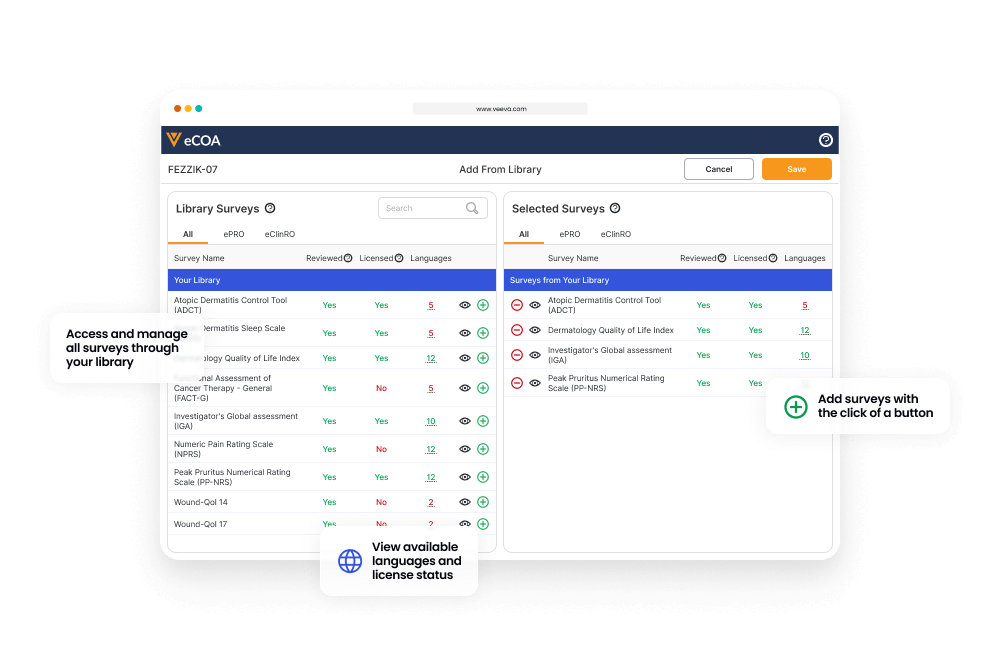
Sponsors manage the eCOAs through their own interface, and a central library allows them to reuse eCOAs across all their studies.
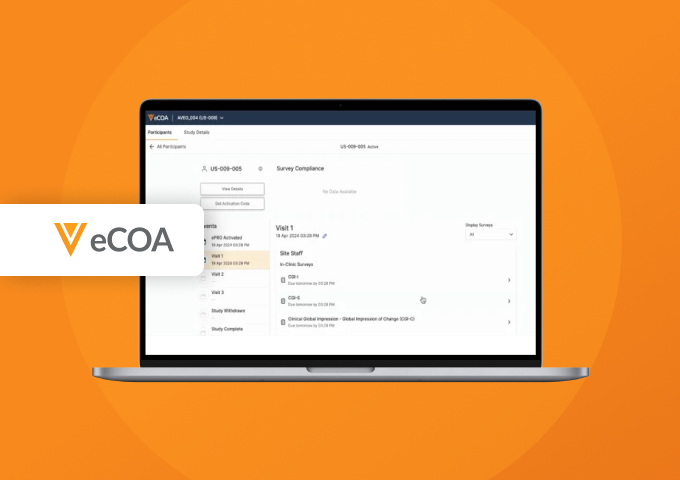
Sites have a simple access point to manage their participants and can review eCOA data and adherence.
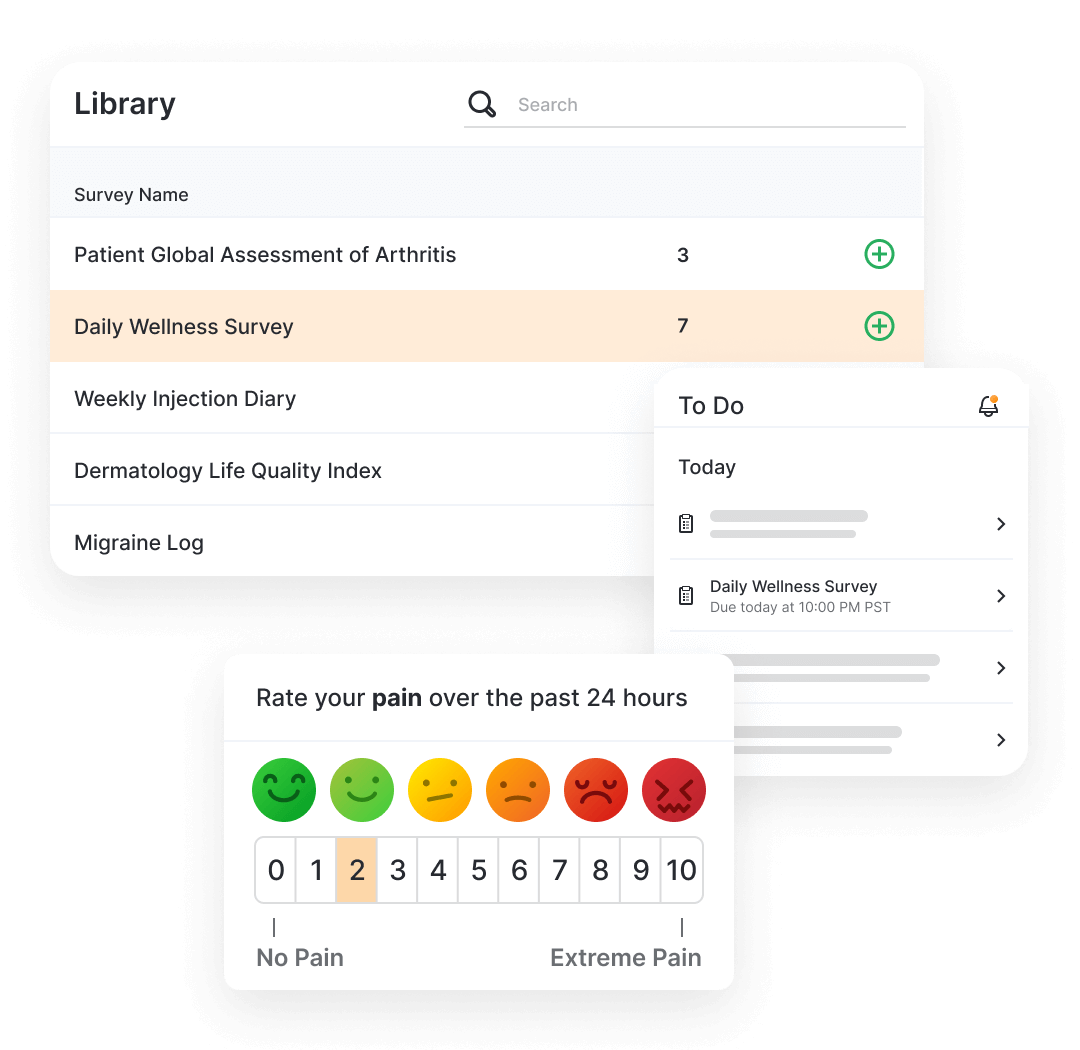
Patients and caregivers complete the questionnaires using MyVeeva for Patients (native application or web), where they can also access other activities like consent or virtual visits. Once complete, the data flows back into the sponsor’s environment.
Announced 2022 Status Early Customers 11-50
6 new standards to simplify eCOA delivery and management
Overview
Deliver eCOA at Scale
eCOA (electronic Clinical Outcome Assessments) captures questionnaire responses directly from clinical trial patients (ePRO), clinicians (eClinRO) or patient caregivers (eObsRO) using an app or webpage.
Sponsors manage the eCOAs through their own interface, and a central library allows them to reuse eCOAs across all their studies.
Sites have a simple access point to manage their participants and can review eCOA data and adherence.
Patients and caregivers complete the questionnaires using MyVeeva for Patients (native application or web), where they can also access other activities like consent or virtual visits. Once complete, the data flows back into the sponsor’s environment.
Why Veeva eCOA
Build studies faster and smarter
Resources
Explore and Learn
Read Features Brief
Veeva eCOA Features Brief

Download Instrument List
Over 200 Validated Instruments Supported for Veeva eCOA

Read Blog
The Case for Early Data Management Input in eCOA
Download White Paper
Raise the Bar: Setting New Standards for the eCOA Industry

View Webinar
A New Approach to De-risk your eCOA Strategy

Watch Demo
Make Taking Part in a Clinical Trial More Accessible and Convenient

Watch Demo
Simplify Site eCOA Completion and Management
Learn More
Deliver a Better Site and Patient eConsent Experience

