White Paper
Building a Compliant Content Foundation for the Future
Over the last decade, emerging biotechs have branched out from historically common licensing, partnership, and acquisition paths to launch products themselves. In fact, industry data estimates that by 2026, first-time biotech launches will outnumber those from established companies by more than two to one.
For many emerging biotechs, preparing for launch means focusing attention on essential foundational content areas. The quality and compliance of the foundation you lay for your content workflows now — including medical, legal, and regulatory (MLR) review processes — can create a competitive edge and help your organization scale more efficiently in the future.
Compliant content management optimizes review and approval
Speed is critical to maintaining launch timelines, but moving too quickly can lead to costly mistakes. In 2023, life sciences companies in the U.S. paid roughly $1.8 billion to settle False Claims Act violations and off-label promotions.
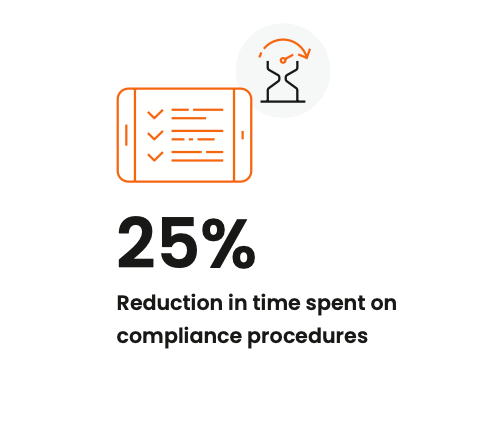
The right foundation for MLR review and approval reduces the risk of fines while increasing speed and efficiency. Along with improved compliance through a clear chain of custody and automated audit trails, Veeva PromoMats users reported a 25% reduction in time spent on compliance procedures.
"We’re trying to be transformative, but we have to lay the foundation first." Robert Yee, Senior Product Manager, Azurity Pharmaceuticals
Fast-track MLR with a dedicated system
Emerging biotechs commonly use email and spreadsheets as a starting point to manage MLR reviews and approvals. However, these systems are highly manual and time-consuming, so it’s easy to lose track of where an asset stands in the review process. The result: a drain on your organization’s limited resources and increased compliance risks.
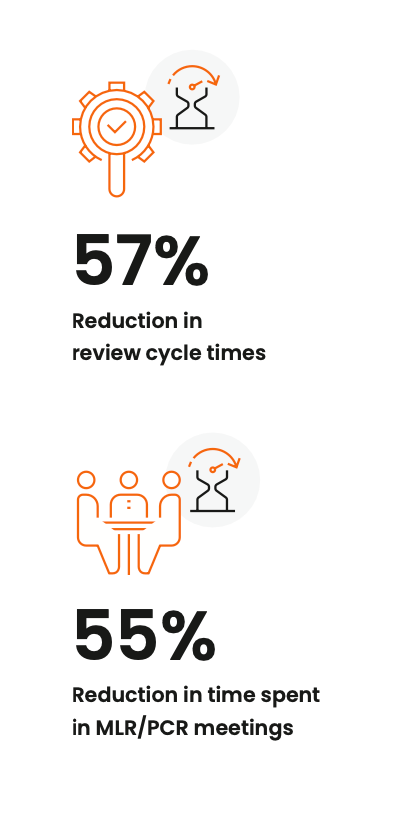
A more sustainable option is to automate these cycles through a dedicated MLR review system. With review and approval tracked in one place, your stakeholders can manage approval queues and gain visibility into content moving down the pipeline. Meanwhile, you can quickly identify and address any bottlenecks in the process. Organizations with streamlined MLR processes in Veeva PromoMats report a 57% reduction in review cycle times and a 55% reduction in review meeting duration, advancing speed and compliance.
Modern claims management enables launch efficiency
Manual processes like tagging and anchoring claims to supporting references are not only time-consuming but also prone to human error. As you navigate peak content volumes and aggressive deadlines during launch, there’s no room for delays. A modern approach to claims management can help your organization avoid slowdowns and maintain compliance with the automation of repetitive tasks, clear audit trails, and traceability.
"By using a core technology solution, we have a single source of truth for approved content and the ability to flex and optimize our MLR workflows as the company evolves." Martin Boyle, Senior Director of Field and Marketing Operations, Regeneron
Use modern claims management to optimize your content operations by automatically tagging and linking materials to approved claims. And when you stand up a central claims library, your stakeholders gain access to standardized language, improved transparency, and accurate documentation.
"80% of our claims are now linked automatically. This automation has significantly cut down manual linking, which used to take 10 minutes per claim, and now constitutes only about 20% of the overall process." Senior Marketing Director, Emerging Biotech
Anticipating and preparing for future challenges
Establishing a standardized content taxonomy from the start
Emerging biotechs that implement a standard taxonomy while building a content foundation can avoid future disconnects with channels, intent, and format. Veeva announced a freely open and available Commercial Content Kernel to help software applications, data products, and people talk to each other with greater consistency and accuracy. It provides a standard content hierarchy, classification, and description for organizing and delivering content.
As an emerging biotech, adopting the Commercial Content Kernel from the start will help you increase speed, efficiency, and quality of data management for your organization in the future. When content operations scale, you’ll be able to locate, manage, and repurpose materials much more easily and efficiently.
Adopting a comprehensive, connected solution
Traditional content methods — siloed systems or paper-based processes — can delay content availability and create compliance risks. The integration of PromoMats with other Veeva solutions, such as Veeva RIM, Medical Suite, and Vault CRM, creates a connected ecosystem that enhances content management across the end-to-end lifecycle.
"With Veeva PromoMats, we can easily collaborate, share, and integrate digital content across our Veeva ecosystem. This drives success right out the gate because we immediately gain valuable insights to inform more efficient content production and can implement changes quickly, as needed." Associate Director, IT Product Manager, Biotech
These connections allow for seamless data flow between different systems, ensuring that content is compliant and of the highest quality throughout creation, review, approval, and distribution.
Schedule a call with Joe Adams, Strategy Director, Commercial Content Europe, to discuss your content strategy, and learn why 350+ emerging and mid-size biotechs use PromoMats to build a compliant content foundation.
