White Paper
How to Maintain Standards for Collecting Clinical Data
As your company grows and runs more trials, reusing case report form (CRF) designs and edit checks can deliver benefits at scale. The increase in efficiency, speed, and quality motivate organizations to develop internal data collection standards1 for their clinical data collection instruments. Yet, these benefits are only possible with consistent enforcement and governance processes in place.
Many data management teams report that their standards initiatives have stalled or failed due to a lack of executive buy-in, an over-proliferation of standards, or standards stuffed with exploratory variables. It is possible to successfully establish internal data collection standards if the right stakeholders are involved, governance is established, and change management is prioritized.
Here is a How-to Guide with best practices and advice for establishing and maintaining a successful clinical data collection standards program.
Setting Up Standards For Success
Establishing standards requires a team effort and organizational buy-in from the very top. While a small working group hammers out the proposed templates or standards, executive support is needed at the beginning to get started in the right direction and at the end for final approvals. Internal alignment is critical to success, therefore be sure to incorporate the needs of downstream teams such as stats programming and statistics. Here are some tips to help teams set-up standards for success.
- Faster builds in your EDC system
- Higher quality data entry at the sites
- Easier and more complete reporting
- More efficient cleaning and transformations
- Freed resources to focus on study-specific matters
Involve everyone with a ‘horse in the race.’ Involve representatives from each stakeholder group to ensure alignment across stats, clinical, medical, and data management. Different groups will have competing interests and therefore should be represented when making compromises and tradeoffs.
Find the right people. When nominating area representatives, consider people’s natural tendencies and inner motivations. Look for individuals with an inclination to organize and classify. Standards projects are almost always done on top of the person’s day job, so look for the unique individual who thinks data standards are cool.
Codify decisions as you go. If your organization doesn’t have standards in place, the easiest and most pragmatic way to get started is to codify decisions as you go and build your library of standards over time. Start capturing key decisions, reasons for each decision, and usage guidance within a simple standards manual and Frequency Asked Questions guide. Learn from others. Your data and technology providers can be valuable partners in creating standards. They can likely offer a starting point based on their own standards, which already reflect lessons from mistakes and successes across multiple clients. Vendors may also have visibility across studies in your organization and can identify commonalities to help formulate your standards.
Learn from others. Your data and technology providers can be valuable partners in creating standards. They can likely offer a starting point based on their own standards, which already reflect lessons from mistakes and successes across multiple clients. Vendors may also have visibility across studies in your organization and can identify commonalities to help formulate your standards.
Defining Data Standards
When it comes to data collection, overall endpoints and objectives are key. What data are necessary to feed your analysis? Standards should only collect what’s required to support your study objectives. Exploratory data is potentially useful, but diverts resources from critical data and places additional burden on patients and sites. Start defining your data standards with these first steps.
Don’t reinvent the wheel. Use industry standards such as CDISC’s CDASH as your foundation. Years of industry collaboration have contributed to their design and they will help ensure traceability and mapping to end deliverables such as SDTM variable names.
Segregate as needed. Different therapeutic areas often have distinct data collection requirements and will need their own standardized forms. When possible, make area-specific forms additive to your corporate standards rather than a variation. Introducing variations is a slippery slope towards version proliferation and a breakdown of control.
Keep standards lean. Placing an item in your standard forces every study going forward to collect that data. Packing CRF standards with exploratory fields will greatly increase data collection, cleaning, and monitoring costs.
Offer “suggested templates” in addition to formal standards. Official standards should be infrequently changed and rigorously enforced. Offering unofficial templates that function as starting points and can be readily changed will increase your efficiency and consistency, while allowing flexibility.
Keep it real. Define standards that capture reality. Limiting your case report forms to collect only what you want to occur and excluding options that would capture unwanted outcomes will result in dirty data and avoidable queries as sites attempt to accurately document what happened.
Don’t stop at CRFs. Standardize the supporting documentation such as the CRF completion guides, test cases, test results, and mappings to transformation programs. When a study reuses collection instruments, they can also reuse the programs that transform the data to SDTM. The time savings generated by reusing supporting documents and downstream programs are critical to making the financial case for standards and securing executive-level buy-in.
Your eCRF standards can be tracked and managed at the top-level, as complete supersets of forms (by therapeutic area or corporate standards), as well as at the individual form level. For example, version 12.0 of a therapeutic area standard may include version 4.0 of the serious adverse event form for that therapeutic area. The superset of eCRF specs can also include standards for default EDC settings, subject numbering guidelines, abbreviations, and other standardized conventions.
Below we provide an example of one company’s spec and the variable table used to provide instructions, variable names, code list values, and more for each data element and unit on the form.
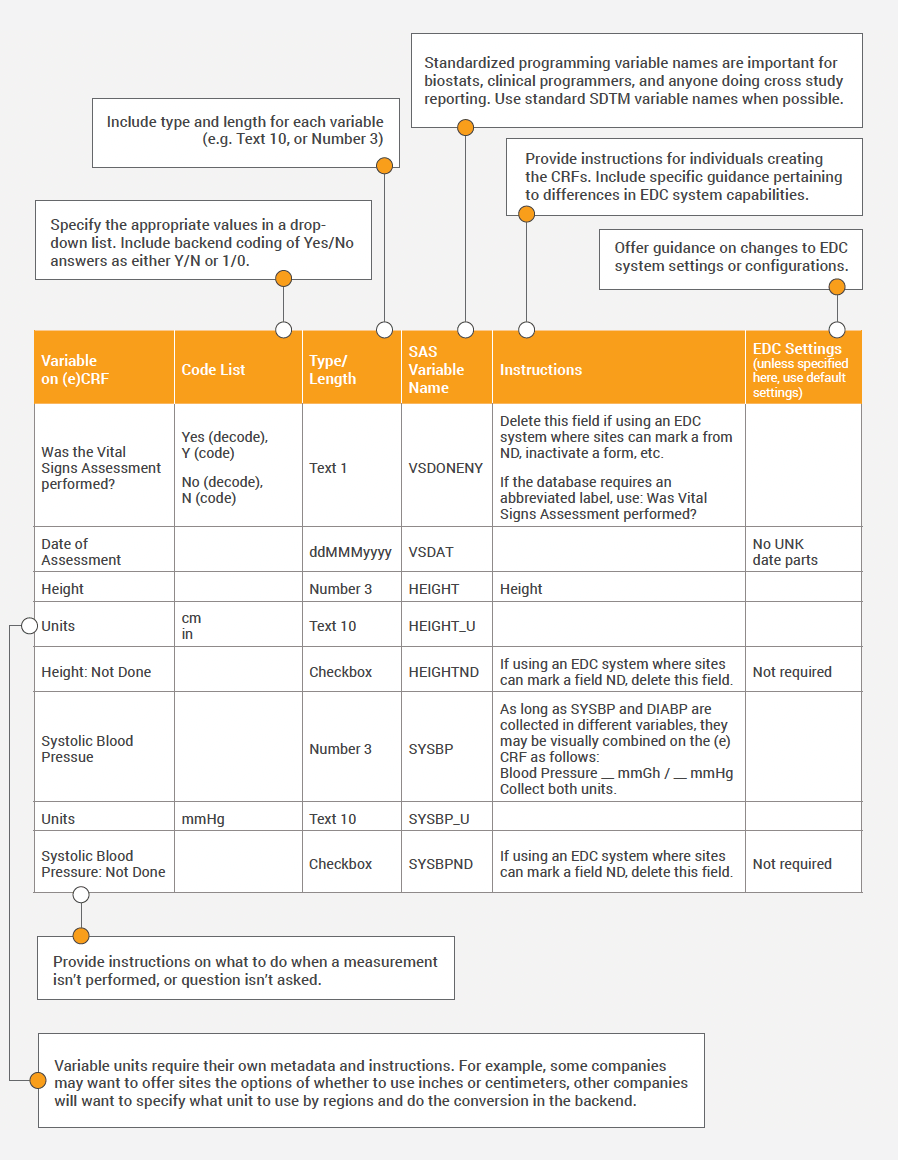
Establishing Governance, Deviations, and Updates
A successful standards program includes a formal change control process whereby individuals can ask for permission to deviate from the standards for their study. Email lacks the tracking and workflows that are necessary for effective management. Any deviation from a standard must be approved by the governance committee. The expected answer to deviation requests should be “no.” Typically only deviations that are necessary for analysis should be approved. Follow these three steps to create an effective governance structure.
GOVERNANCE STRUCTURE
Executive committee to maintain corporate commitment. The executive committee provides oversight and approves new standards and changes to standards. A strong, cross-functional executive committee is crucial to maintaining corporate buy-in. Without executive-level commitment, powerful study team members will persuade their teams to change, deviations will become common, and standards will fail to deliver the promised benefits.
Governance committee(s) to manage change requests. A manager-level governance committee does most of the standards work and has three main functions: approving/denying deviation requests, developing new standards, and reviewing existing standards on a periodic basis to evaluate whether updates are needed. The governance committee evaluates requests to deviate from a standard; and needs a direct line of communication into other teams such as Stats Programming to ensure they fully understand the impact of the deviation before making their decision. Committee decisions should be documented and communicated to downstream study team members and supporting vendors.
Separate committees or sub-committees may be needed for different therapeutic areas in order to provide sufficient domain experience, agility, and scale.
Updating your standards. Making updates to the standards themselves is an infrequent but important part of a healthy standards program. Updating standards on an annual or biannual basis is a reasonable and achievable cadence; and is only needed for those standards with frequent deviation requests. This is where tracking deviation requests and approved deviations is critical to making informed decisions and maintaining the organization’s confidence in the quality and relevance of its standards.
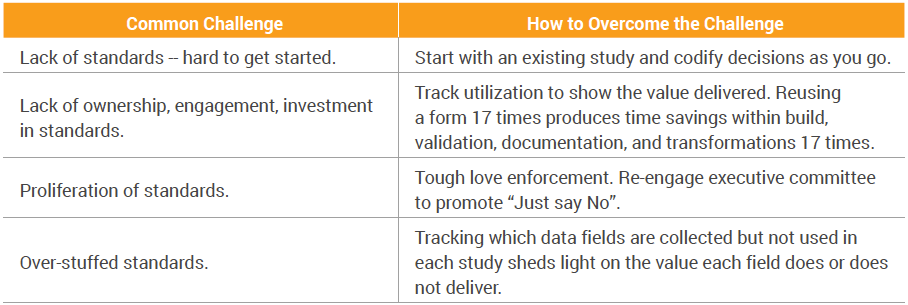
| Common Challenge | How to Overcome the Challenge |
|---|---|
| Lack of standards -- hard to get started. | Start with an existing study and codify decisions as you go. |
| Lack of ownership, engagement, investment in standards. |
Track utilization to show the value delivered. Reusing a form 17 times produces time savings within build, validation, documentation, and transformations 17 times. |
| Proliferation of standards. | Tough love enforcement. Re-engage executive committee to promote “Just say No”. |
| Over-stuffed standards. | Tracking which data fields are collected but not used in each study sheds light on the value each field does or does not deliver. |
Fostering Adoption and Use
Many standards initiatives fail to deliver the promised efficiencies because deviation requests were too readily approved, or study teams proceeded independently without regard for the process. Therefore, fostering adoption of the standards across your organization is crucial to the success of your initiative. Everyone involved with a study should understand the value of data collection standards. Prioritize change management by focusing on these three areas.
FAQ documentation. An FAQ or similar standards policy document should outline what types of changes are acceptable and what changes are not allowed. Documenting the changes that aren’t allowed, along with the supporting rationale, will decrease the burden of superfluous requests on your governance committee.
Onboarding and training. New employees arrive with their own perspectives, approaches, and habits established at prior employers. To ensure alignment and consistency, standards training should be mandatory for every new employee hired within the Development organization.
The curriculum should include what the standards are, the organization’s philosophy on standards, the areas covered by standards, and the benefits the organization achieves. Set expectations that standards have been established for a reason, that pre-approved deviations or variations of a standard are documented, and that requests to diverge from standard will be declined except in rare circumstances.
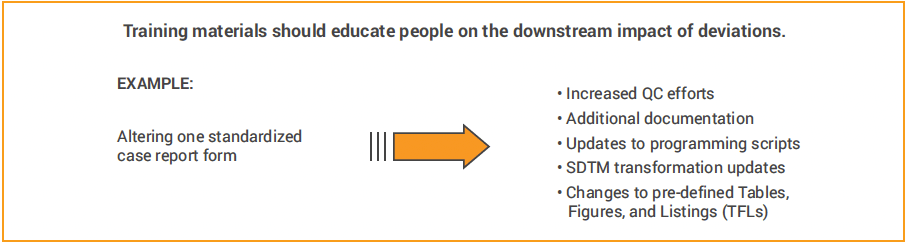
Consistent enforcement. Leverage members of your data management team to perform quality control checks. These individuals should look at new studies to double-check that the CRFs, data exchange specs, and other documents are following the standards.
Collaborating with Technology Providers
Technology partners are an important part of implementing any standards program. Sponsors must own the adoption and utilization of standards, but it is the vendors who often implement standards and leverage them when configuring systems, integrations, or case report forms. Here are three steps to maximize partner relationships.

Incorporate vendor expertise
Vendors know their own systems and can provide valuable insights and advice on the optimal means to accomplish your goals.
Enlist vendors in enforcement
Provide vendors with your standards. When they see a deviation, they should ask to see the approval form from the governance committee.
Point of contact
Give vendors a point of contact for communicating requests to deviate from standards.
Adopting a new system is a good time to re-evaluate your standards. New systems can introduce new capabilities or more efficient ways of working. You don’t want to miss out on efficiency gains because standards tie you to dated processes.
Establishing and Maintaining Internal Data Standards
A successful standards program can produce significant efficiencies for your clinical team. Standards deliver faster builds in EDC systems, higher quality data entry at sites, easier and more complete reporting, more efficient cleaning and transformations, and simplified data sharing and analysis across sources and studies. The benefits achieved play an important role in speeding medicines to patients.
Learn how Vertex Pharmaceuticals Inc. cut database build timelines by 50%.
1 The term “standard” has different meanings in different organizations. For the purpose of this paper we consider it the documents, data, and processes established by the organization to serve as a model or formal guideline.
