Veeva eTMF
Improve Trial Efficiency and Stay Inspection Ready
eTMF is the leading trial master file application used to ensure the quality, timeliness, and completeness of a TMF. It provides full enterprise content management capabilities for upload, version control, QC/ approval, and real-time co-authoring with Microsoft Office for study documents such as consent forms. eTMF is highly efficient and supports global outsourcing.
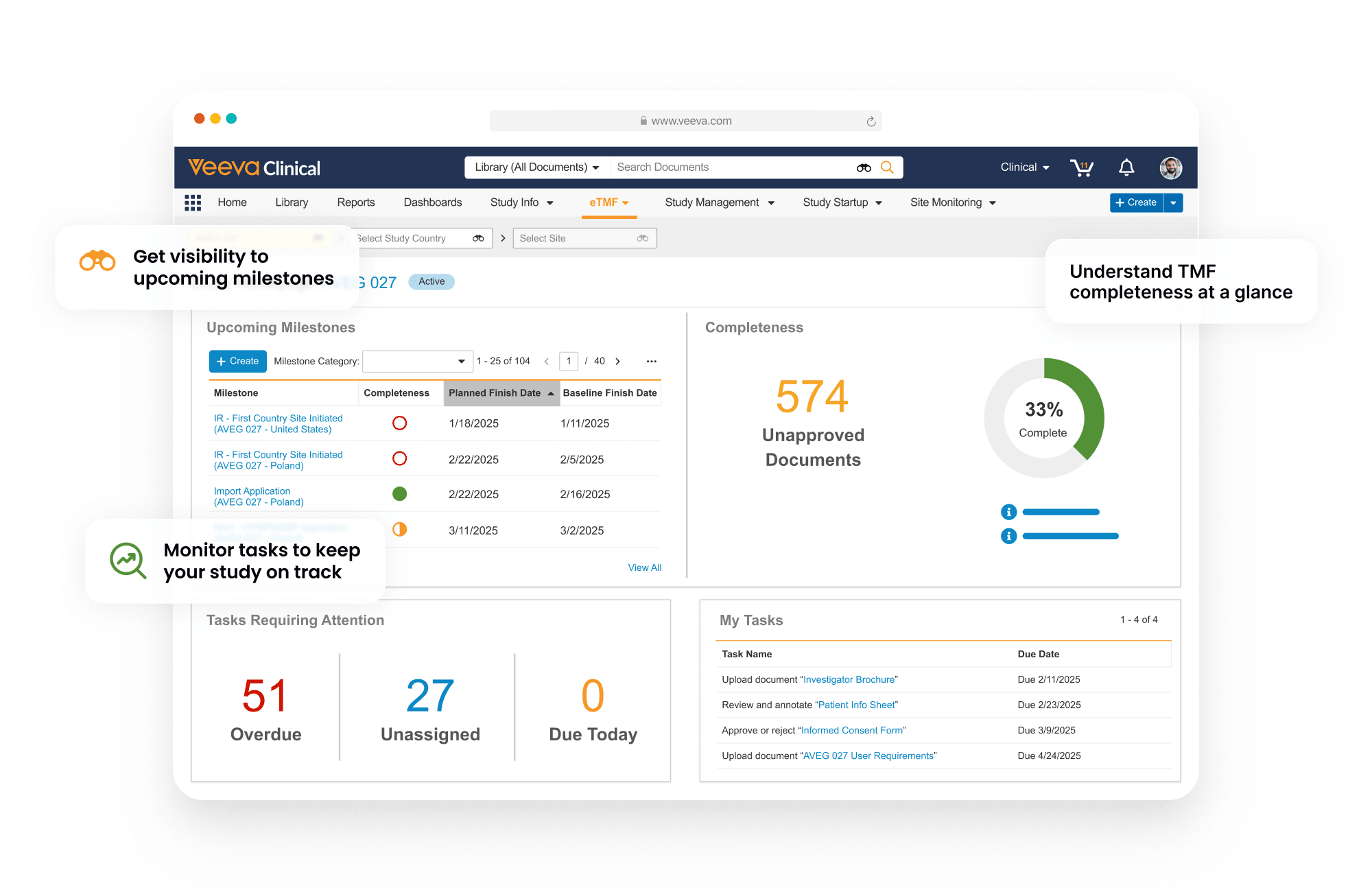
Completeness and timeliness are managed through Expected Document Lists (EDLs). Content files are auto-classified and matched automatically to EDLs.
Risk-based document QC streamlines the document quality control process by assigning a risk level to each document type and applying a specific sampling percentage. When a new document is added to a workflow, the system automatically determines whether a quality check is required, providing traceability to support the audit trail.
The TMF Transfer feature simplifies exchange between sponsors and CROs by sending completed TMFs at study close.
Announced 2012 Status Very Mature Customers 100+
See how 500+ organizations address critical challenges with Veeva eTMF innovations
Overview
Improve Trial Efficiency and Stay Inspection Ready
eTMF is the leading trial master file application used to ensure the quality, timeliness, and completeness of a TMF. It provides full enterprise content management capabilities for upload, version control, QC/ approval, and real-time co-authoring with Microsoft Office for study documents such as consent forms. eTMF is highly efficient and supports global outsourcing.
Completeness and timeliness are managed through Expected Document Lists (EDLs). Content files are auto-classified and matched automatically to EDLs.
Risk-based document QC streamlines the document quality control process by assigning a risk level to each document type and applying a specific sampling percentage. When a new document is added to a workflow, the system automatically determines whether a quality check is required, providing traceability to support the audit trail.
The TMF Transfer feature simplifies exchange between sponsors and CROs by sending completed TMFs at study close.
Veeva AI for Clinical Operations
See in Action
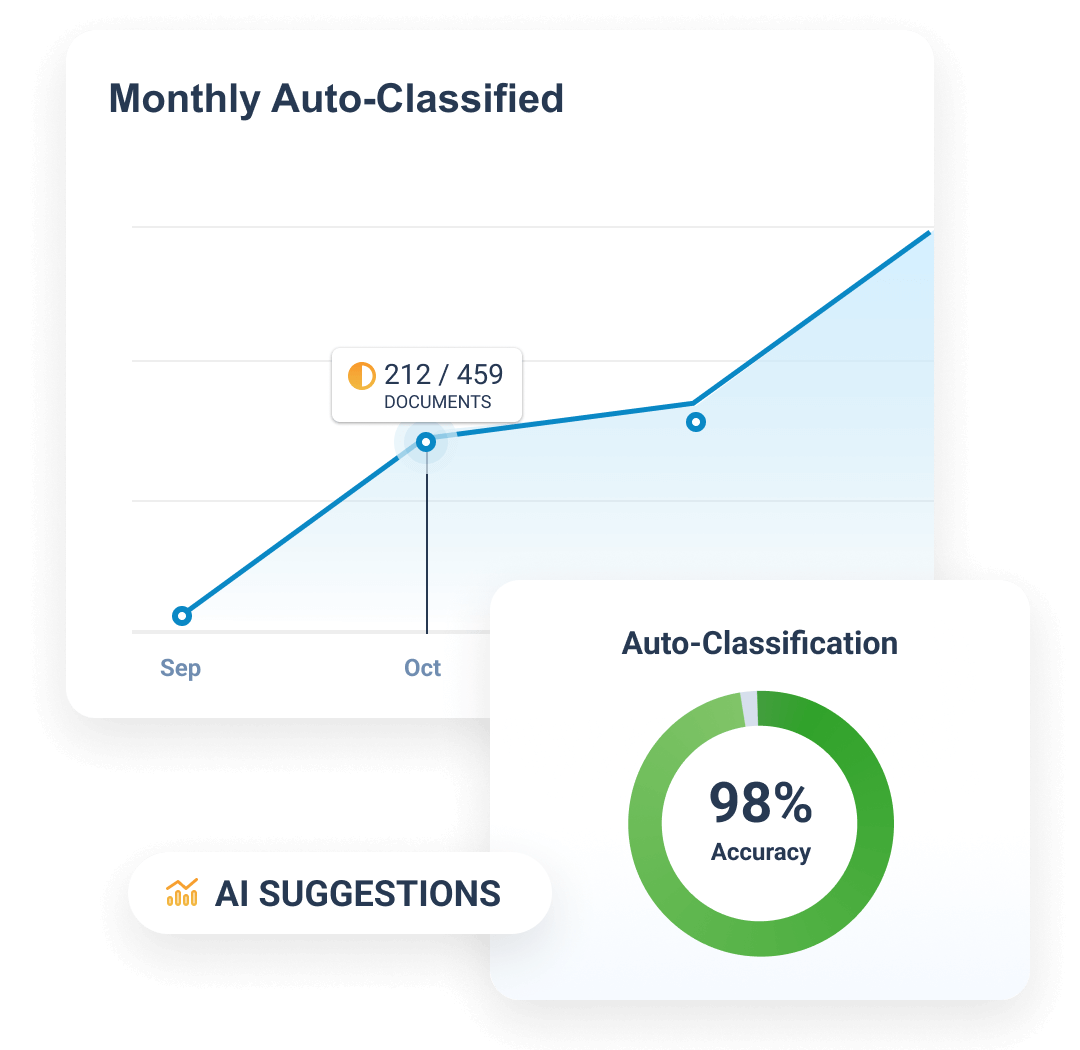
TMF Intake Agent
Automatically classifies, indexes, and adds key metadata to incoming TMF documents, reducing manual work and improving TMF quality.
Quality Check Agent
Reviews a single or set of documents for completeness and accuracy, improving content quality and supporting TMF readiness.
Impact
Efficient and compliant trials
>80%
decrease in TMF migration prep time
75%
faster TMF delivery in outsourced models
40%
cut in study reconciliation time
Why Veeva eTMF
Innovations to drive efficiency and quality
Customer Success
Trusted by 500+ customers to
automate and ensure TMF quality




















Resources
Explore and Learn
Read Features Brief
Find Veeva eTMF Features to Drive TMF Health

Read Report
6 Trends Driving TMF Strategy: From Big Data to TMF RM V4

Watch Customer Video
Moderna Leverages eTMF to Enable Automation and Connectivity

Read Case Study
UroGen Pharma Streamlines Clinical and Regulatory Operations

Watch Demo
Quickly Transfer TMF Documents and Eliminate End-of-study Migrations

Watch Demo
Gain Important Information About Inspection Readiness on the Veeva eTMF Homepage
Read White Paper
Create a Culture of Quality with Veeva eTMF
Get Checklist
Learn Key SOP Sections and Other Tips to Prepare for Inspections
