How One Top 20 Pharma Is Driving Operational Process Improvements
As the hub of clinical operations, it is common for a clinical trial management system (CTMS) to have many integrations with other clinical systems and to be deeply embedded across an organization. One top 20 pharma company had a complex multitude of loosely integrated systems, but prioritized digitization to improve the drug development pathway and value chain. Standardizing on a modern CTMS and unified clinical platform enabled it to achieve significant business benefits.
Recent research shows that an overwhelming majority (99%) say bundling CTMS with at least one other clinical technology is critical.1
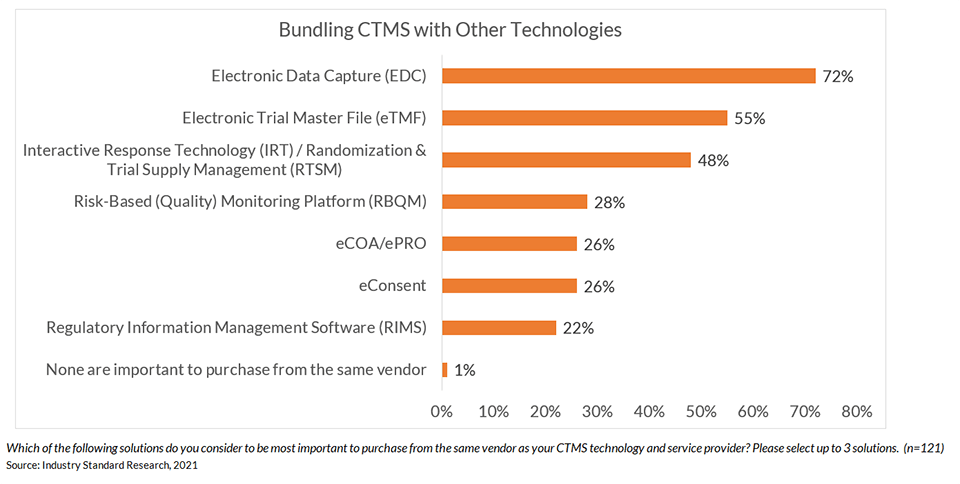
That’s why ease of integration with eClinical technologies is the most important criteria for CTMS solutions, followed by ease of use for clinical sites and sponsor/CRO study teams.2 Seamless interoperability with other clinical systems is necessary to provide complete visibility and oversight across the end-to-end trial process. There is a tremendous opportunity to reduce system and process silos that limit visibility, quality, and speed.
Providing an easy-to-use, intuitive experience across all clinical operations processes boosts productivity and speeds study execution for the top 20 pharma company, driving exceptional results.

Learn more about this pharma’s modernization approach, and how system unification and end-user ease of use are helping it to drive operational process improvements.
1 Source: Industry Standard Research, 2021
2 Source: Industry Standard Research, 2021
