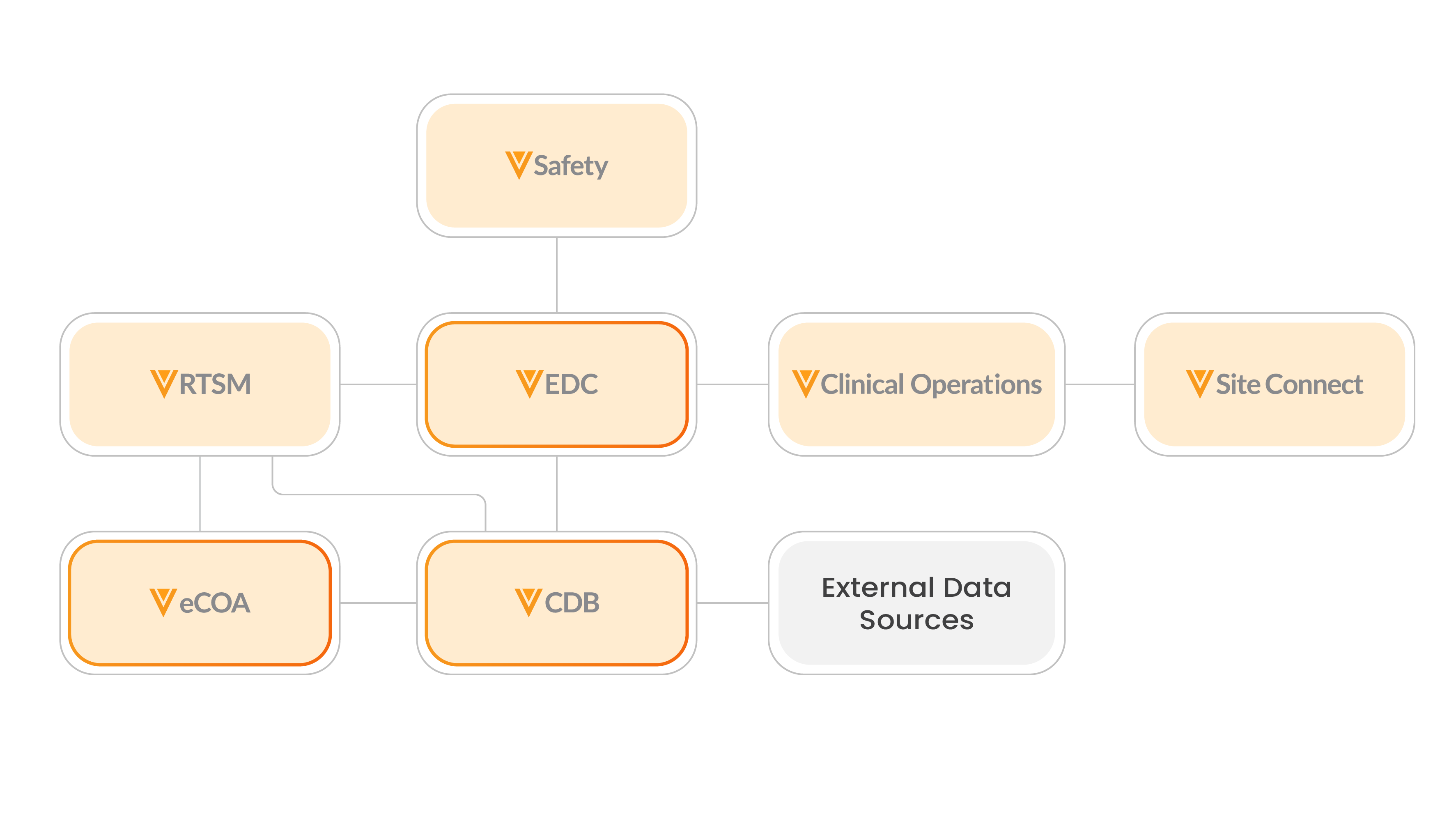
Learn more about Veeva Connections that seamlessly transfer data and documents between Veeva Clinical Data products:
- Clinical Operations-EDC Connection
- Safety-EDC Connection
- RTSM-EDC Connection
- Safety-Clinical Operations Connection
- RTSM-Clinical Operations Connection
- Clinical Operations-eCOA Connection
Veeva Clinical Platform simplifies and standardizes clinical trials to provide:
- Improved efficiency for sponsors
- Reduced complexity for sites
- Better experiences for patients
Watch an end-to-end demo to see the full lifecycle of a safety case as it flows through Veeva’s connected applications.
“Integrating these systems has allowed us to streamline processes, reduce errors, and improve overall efficiency, which is crucial for scaling our operations as we grow.”
Bonne Adams, Vice President of Operations at Inhibrx Therapeutics in Clinical Trial Vanguard
See how biopharmas like Merck, Bayer, and Boehringer Ingelheim improve end-to-end efficiencies with a connected Clinical Platform.
Read blog
Read blog
See how critical study data flows between Veeva Clinical Data products for increased efficiency and less effort.