Improving Medtech Quality by Leveraging Purpose-Built Applications
Confusion abounds at device companies around using product lifecycle management (PLM) for quality functions. The answer lies in leveraging a purpose-built electronic quality management system (QMS) for quality management and processes.
By Christine Kattappuram
These are busy times for life sciences and medtech organizations as market conditions and regulatory changes keep leaders on their toes. More than 70% of companies are undergoing digital transformation with software investments estimated to reach $700 billion. Even with this rise in investment, confusion remains around using product lifecycle management (PLM) for quality functions. An electronic quality management system (QMS) is designed for specific quality processes and would be a natural fit for medtech companies looking to modernize quality and manufacturing. Here are insights into why using PLM for quality is problematic and how PLM and QMS together can establish sustainable, scalable, and advanced quality operations.
Why Using PLM for Quality Processes Doesn’t Work
PLM and QMS solutions each have unique use cases. PLM solutions are engineering-focused and used for new product designs, parts, and bill of materials management. They allow companies to work on products from concept to retirement. A QMS is designed to manage quality documents, employee training, product complaints, nonconformances, corrective and preventive actions (CAPA), supplier quality, and audits as global regulations require.
Companies may be encouraged to “build in quality” during product design by using a PLM solution as part of a one-size-fits-all strategy that reduces upfront software costs and maximizes ROI. But PLM has a specific use and leveraging it to manage quality can lead to consequences in a highly regulated industry such as medtech. This is a risk most companies can’t afford, especially since the FDA recalled 57 medical devices in 2021, a 50% increase over the prior year.
Using PLM might seem appropriate for specific quality processes like engineering change orders and requests, but this approach leads to highly customized, burdensome-to-maintain system designs. Medtech companies can quickly find themselves working with inflexible solutions that cannot adapt to business or regulatory requirement changes.
“Organizations using a highly customized PLM system have experienced challenges with interoperability, data structure, and lack of efficiency,” said Hayden Atkinson, global enterprise systems compliance manager at CooperVision. “It is a priority to modernize by leveraging purpose-built QMS applications so medtech companies can focus on building their products rather than customizing IT applications to meet their quality needs.”
Utilizing technology applications outside their intended purpose dilutes value and exposes medtech manufacturers to significant risk. As of today, the PLM systems available for medical devices were initially designed to serve the needs of the automotive and aviation industries. These solutions require a lot of management and attention and lack connectivity with other systems, making it harder to access data and generate reports.
PwC partner Kareem Elwakil offers some advice for medtech organizations. In his view, “Using PLM for core quality functionality sets companies on a complex, slippery path. These systems don’t have the depth or breadth to manage quality at their core. This space requires process expertise and knowledge of the evolving global regulatory landscape, making QMS a better fit for medtech companies.” The promise of expanding the use of an existing PLM solution may create short-term savings, but the reality is the long-term costs add up quickly because of complex customizations. Steve de Baca, executive vice president of QA/RA at Cardinal Health, recently told quality leaders at a roundtable, “Don’t get enamored with the day one cost. Focus on the total lifetime cost, maintenance, and changeability.”
Remember that customization is not the same as transformation. When organizations modify solutions for processes outside their intended uses, costs and complexity increase. This hinders innovation, limits compatibility with future software updates, and makes managing validation and release cycles challenging.
Unlocking the Benefit of PLM and QMS
To achieve true digital transformation, advance to a more modern, connected approach. Take advantage of the opportunity to think creatively and critically about any inefficiencies. Digital transformation projects will quickly expose poor processes, so it is critical to lay the proper foundation for maximizing success.
Next, identify the optimal platform architecture while limiting customization. When organizations consider investments in digital systems, PLM and purpose-built quality solutions should be evaluated together to build a connected technology landscape. Each should have well-defined use cases and deliver the ability to integrate for end-to-end process execution. (See example in Figure 1.)
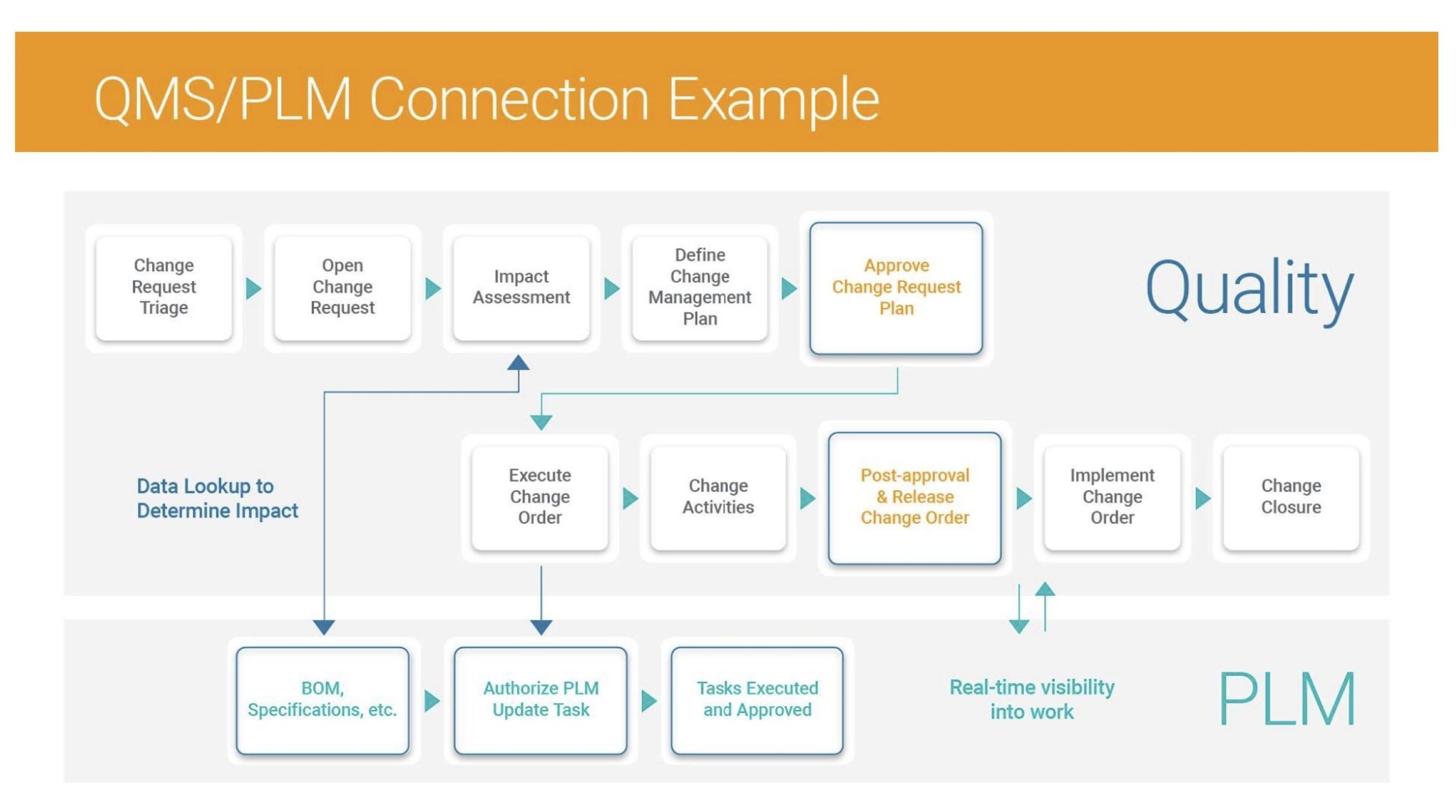
Source: Veeva Systems
Investing in modern technology built on multi-tenancy platforms will minimize technology debt and increase security. Multi-tenant SaaS architecture provides easy onboarding, streamlined maintenance, scalability, and larger computing capacity. This makes a multi-tenant architecture a wise, long-term investment that can be expanded to fit the needs of any organization.
As the medtech industry continues to advance digital transformation initiatives, quality leaders should begin with a clear vision and strategy. Next, identify technologies that align with the specific processes and areas they want to transform. Adopting purpose-built applications on a modern architecture can empower organizations to drive more innovation faster to help patients lead longer and healthier lives.
About the Author:
Christine Kattappuram is senior director of quality strategy at Veeva MedTech and has more than 15 years of experience in healthcare and medical devices, helping companies manage and implement operations and growth strategies. She can be reached at christine.kattappuram@veeva.com.
This article originally appeared on MedTechStrategist.com.