RIM Buyer’s Guide for Medtech
With an increasing amount of global regulatory complexity, regulatory affairs teams are being asked to handle more submissions than ever with minimal resources. Current efforts are not sufficient to keep up with the rising demand, and the result is disjointed data and siloed processes. As illustrated in a recent regulatory benchmark survey, only 14% of participants reported that they had a single source of truth for strategic plans and a mere 13% had a collaborative system to assess the impact of new regulations.
Many companies are now turning to regulatory information management (RIM) systems to centralize information, streamline operations, and accelerate regulatory timelines while maintaining compliance. This buyer’s guide is designed to help teams find the right RIM solution to best meet their needs.
Determining scope and support
First, it’s important for regulatory affairs teams to assess current ways of working and identify key areas of improvement. Are you facing fines for non-compliance or missing renewal deadlines? Does your team waste time and resources trying to track down critical documents? What are the direct and indirect costs for these shortfalls?
Once you understand the gaps, then you can start to gather support for a new solution to meet those needs. If your company is already undergoing a digital transformation project, you may be able to dovetail efforts. If not, then you’ll want to dig into the internal process for purchasing software. Is a separate budget required? Who needs to be involved in the final decision? Are there champions that you can loop in along the way? Remember that it’s not just regulatory leadership signing off on a new system; you’ll need IT leadership on board as well.
Evaluating software capabilities
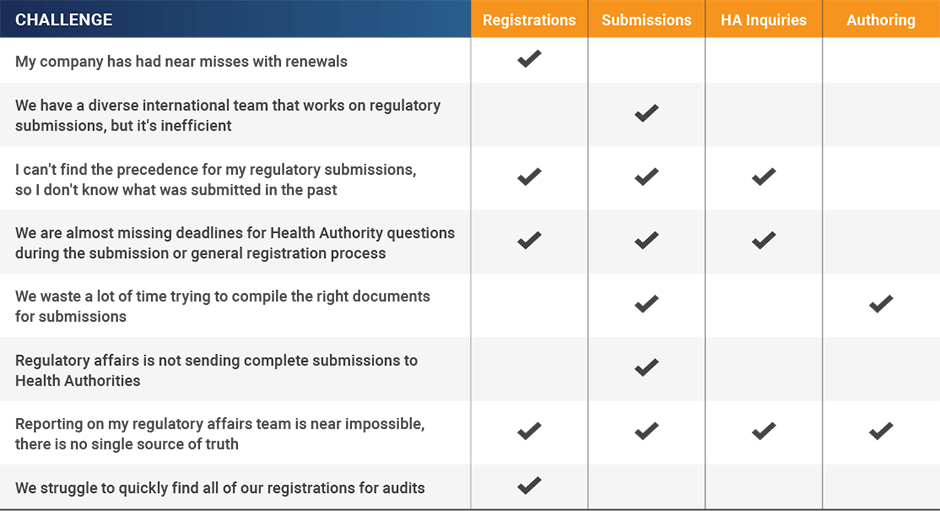
After outlining the project and gaining support, you can start to match your pain points with specific RIM software capabilities. Use the table below to determine if you need a solution that supports registrations, submissions, health authority inquiries, authoring, or a combination of all four areas.

Balancing cost and value
Implementing a RIM solution can drive tremendous value by easing the administrative burden on regulatory affairs and facilitating processes like:



As you go deeper into the buying process, it’s important to evaluate other potential benefits. Will this new solution reduce the time it takes to register products? Will you now have the information you need to answer questions about where products are registered? Can you shift away from administrative tasks and refocus efforts on more strategic projects like adding markets to your product’s global expansion strategy?
Once you have a solid grasp on the value a RIM system can provide, you’ll need to reconcile that value with solution costs. It’s important to remember that not all pricing sheets are created equal. If a software partner doesn’t have a proven track record of starting projects on time or delivering planned functionality, the true cost of implementation may actually be much higher. On the flip side, if the partner offers additional services like business consulting, you may be able to optimize steps such as data migration and change management to take the burden off internal teams. Leading with best practices can help reduce costs in the long term.
Another factor that companies should consider is ongoing support. Are there regular updates built into the RIM software and how is that information communicated? Will you have the necessary resources to configure and support new functionality? Is there a user community that you can tap into for questions or advice?
Finally, medtech companies should review use cases that span multiple domains and require cross-team collaboration. Other stakeholders will undoubtedly need access to this information, so you’ll want to think through integrations with internal systems to form a complete ecosystem.
Answering these questions will help you prioritize solution capabilities and select a solution that’s fit for purpose. To get started, download the RIM buyer’s guide worksheet.