
Terumo uses Study Training to manage training requirements for staff, sites and CROs to improve compliance
View Customer Story
Boston Scientific discusses the value of unifying clinical operations and change management to harmonize processes
View Customer Story

B. Braun shares how clear SOPs and a governance model led to a successful go-live with Veeva eTMF, CTMS, and Payments
View Customer Story
Efficient and compliant training
Veeva Study Training manages GCP and study-specific training for research sites, CROs, and sponsor personnel. It provides document, video, and training modules, in addition to quizzes and classroom capabilities based on curricula and training requirements.
Teams can create a protocol-specific curriculum, which automatically assigns training based on a user’s role and location. Completed training is documented automatically in an inspection-ready format for study teams and CRAs to leverage.
Veeva Study Training connects to Veeva eTMF to eliminate the need to manually capture study and site information.
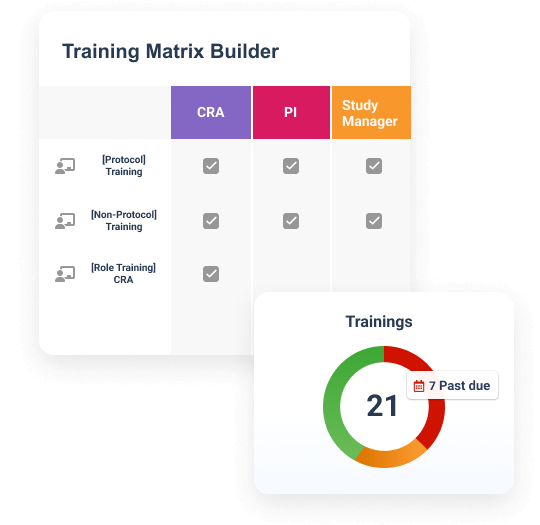
Veeva Study Training Impact
Simplify training with one application
Create, assign, and deliver role-based training for sponsors, CROs, and sites in one solution.Ensure inspection readiness
Ensure compliance with automated training alerts, on-demand content, and real-time visibility.Improve compliance for study teams
Ensure compliance with automated training alerts, on-demand content, and real-time visibility.Drive oversight of study partners
Leverage unified reporting across sponsors, CROs, and sites to improve oversight.
See Veeva Study Training in action
Customer Success
Medtechs speed trials with Veeva Clinical Operations
































Watch video
Learn how Terumo uses Study Training to manage training requirements for staff, sites and CROs to mprove compliance

Watch video
Boston Scientific discusses the value of unifying clinical operations and change management to harmonize processes
Read article
B. Braun shares how clear SOPs and a governance model led to a successful go-live with Veeva eTMF, CTMS, and Payments

View infographic
The MedTech Clinical Benchmark examines how companies are increasing trial activity to maintain product marketability

Watch video
Radiometer shares how a unified clinical platform improved collaboration to scale clinical study volume by 400%

Read article
Terumo partners with Veeva Business Consulting to execute their 3-phase organizational change management strategy



