Deliver eCOA at Scale
Announced 2022
Status Early
Customers 11-50
Veeva eCOA (Electronic Clinical Outcome Assessment) captures questionnaire responses directly from patients and clinicians to ensure consistent and real-time tracking of success criteria.
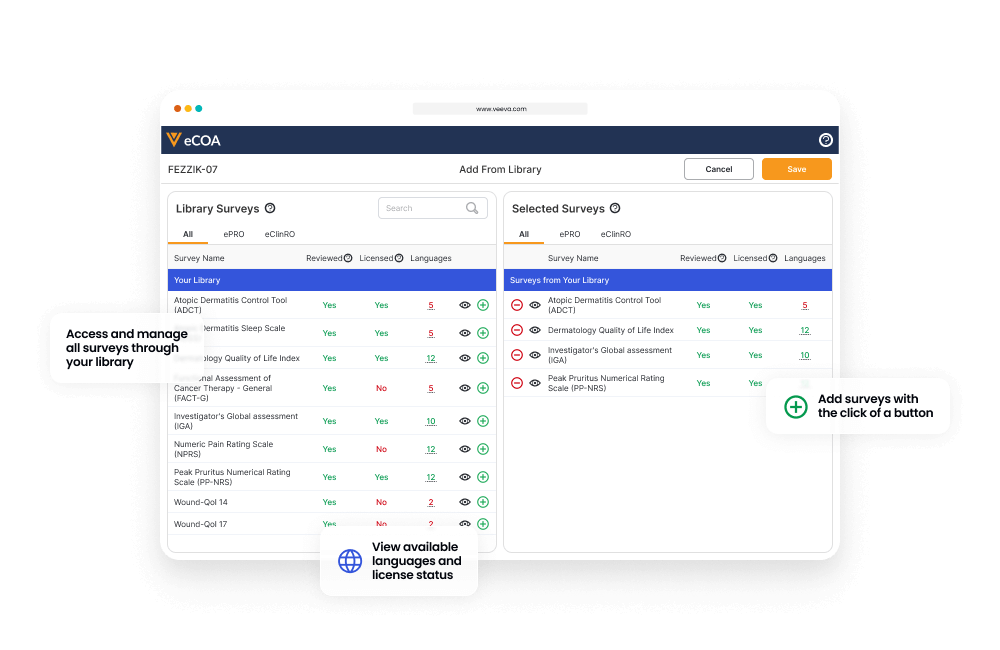
Sponsors manage eCOA submissions from patients and sites with a central library that allows them to reuse eCOAs across all their studies.
Sites manage participants and review eCOA data and adherence for improved communication with Sponsors.
Patients can access virtual visits, consent, and complete documentation using MyVeeva for Patients. All data is captured and shared back to the sponsor’s central library for ongoing tracking.
Veeva eCOA Impact
Accelerate study design and execution
Build, manage, and change studies faster with a modern designer and full reusability across studies.Access high-quality data faster
Ensure data is accurate, complete, and readily available from study design to close out.Reduce site burden
Simplify the setup, collection, and real-time review of eCOAs for sites.Improve patient experience
Make eCOA completion easier with a single user-friendly app to access all study activities.
See Veeva eCOA in action
Customer Success
Medtechs manage complex trials with Veeva Clinical Data
































Read features brief
Veeva eCOA simplifies the design, management and completion of clinical outcome assessments

Watch video
Convatec shares how they implement ePRO to simplify study builds, reduce n costs, and cut study go-live time

View infographic
The Clinical Benchmark studies how organizations manage clinical processes, study site collaboration, and trial data

Read article
Alcon and Illumina share insights on the challenges, best practices, and outcomes of implementing Veeva EDC

Watch video
Learn how Integra unifies their clinical platform to establish repeatable processes and provide data visibility

Watch video
Alcon enables sites to enter data at a median rate of 1.8 days and over 90% of data entered in less than 5 days







