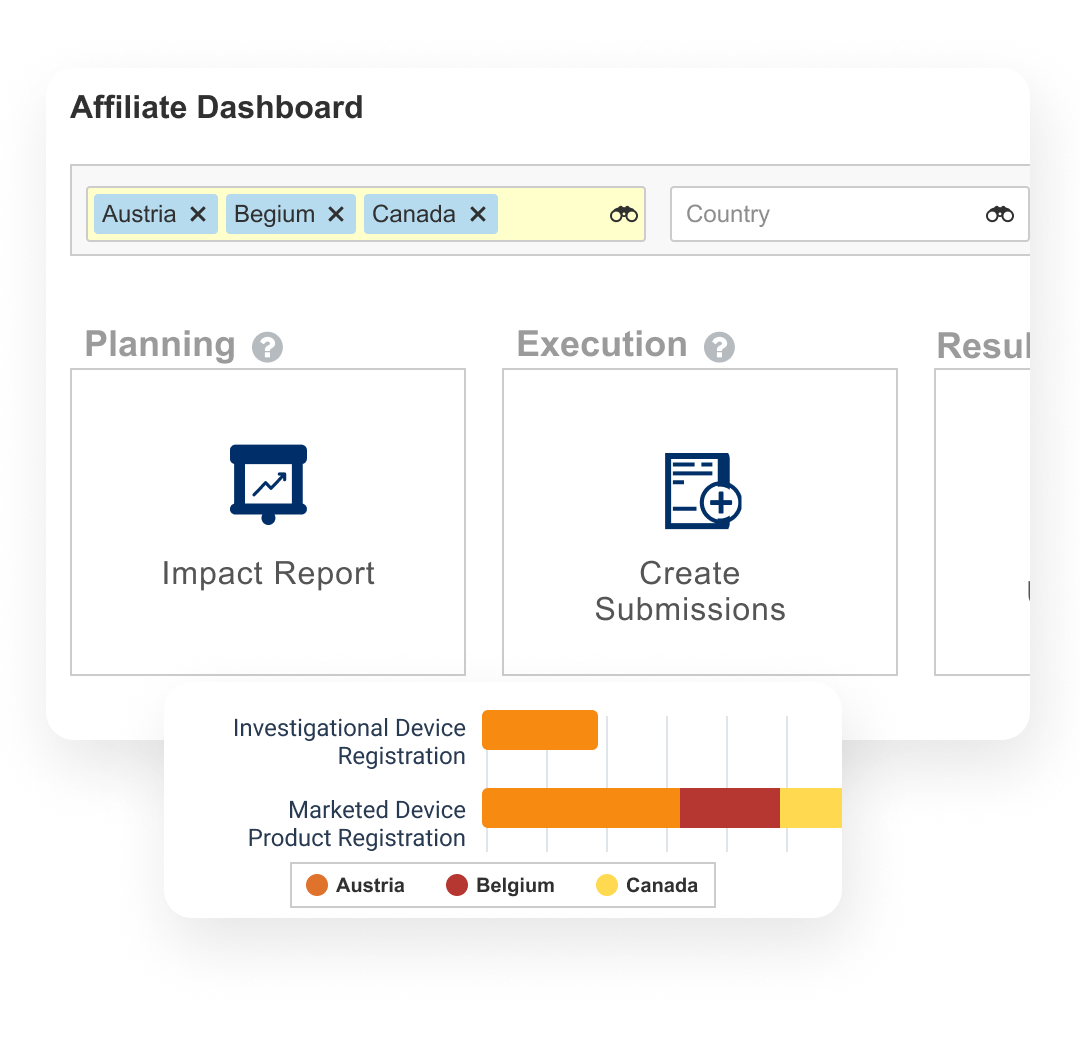
Plan, track, and report on product registrations
Veeva Registrations allows medtech companies to plan, track, and report on global product registrations along with health authority correspondence and commitments.
Events provide the ability to manage product changes, from the initial assessment of the proposed change through submission creation, health authority interactions, and final registration update. Label changes can be tracked and managed at both the global and local level. Registrations also produces compliant product data output for EU regulations.
Dashboards and reports allow personnel to track the progression of change events and provide an understanding of product registration locale.
A real-time regulatory information feed, through our partnership with leading analytics provider Redica, turns data into actionable regulatory intelligence in the context of your portfolio.
30%
more efficient registration management
93%
faster impact assessment creations
95%
less time to update country records
Veeva Registrations Impact
Improve data quality
Streamline registration management by reducing data duplicates and discrepancies.Provide global visibility
Stay informed with a complete view into the marketing status of your global product portfolio.Speed health authority responses
Manage product registration queries and commitments to stay ahead of response deadlines.
See Veeva Registrations in action
Customer Success
Medtechs ensure compliance with Veeva RIM





Read article
Zeiss discusses EU AI Act's impact on medtech, from real-world evidence to content generation

Read white paper
Leading medtechs use RIM solutions to scale efforts, accelerate timelines, and maintain compliance

Watch video
Siemens Healthineers leverages regulatory intelligence to better understand new laws and standards

Learn more
Veeva Registrations Features Brief


