Terumo Unifies Global Clinical Trials to Ensure Standardization and Collaboration
Historically, Terumo’s subsidiaries in the U.S. and Europe designed and executed their own regional clinical trials. The teams used manual and paper-based processes to oversee document control, payments, monitoring of study progress, and visit reports.
With the advent of the EU Medical Device Regulation, the company needed to handle a growing number of clinical trials while complying with new stringent data and reporting requirements. Terumo realized that the current approach was no longer viable in this complex, dynamic environment. Clinical leaders realized the need for a digital platform that could enhance collaboration, efficiency, and ensure global standardization.
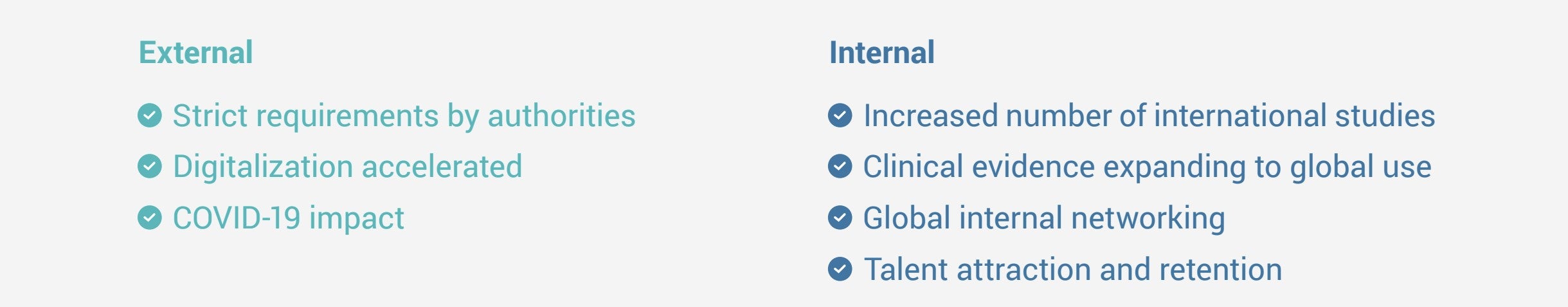
Drivers Impacting Clinical Operations at Terumo:
Global Approach to Digital Transformation
The status quo became untenable gradually, then suddenly. Several factors made it critical for Terumo to have a digital solution, including the increased volume of international studies, the requirement for global clinical evidence, heightened regulatory demand for transparency, and closer internal collaborations.
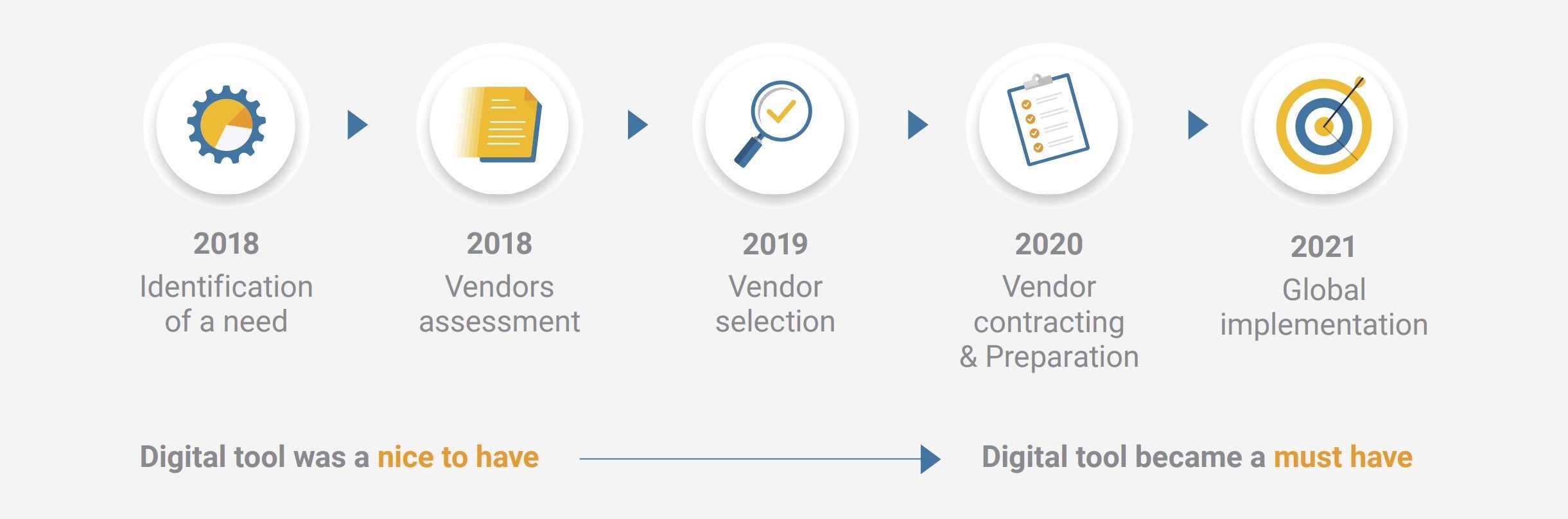
Kazuhisa Senshu Ph.D., chief clinical and regulatory affairs officer at Terumo, recalls: “Initially, a digital tool was a ‘nice to have’ as my team could manage all clinical operations and inspections without eTMF or CTMS.”
He continues, “Then, in 2020, everything changed. COVID-19 had a huge impact, catalyzing remote and decentralized working practices, so a digital solution became a must-have for us.”
With the medical devices landscape changing rapidly, Terumo’s leadership team recognized the need to scale up and streamline its clinical operations capabilities globally. The main objective – boost research efficiency while enabling more robust governance.
Digital Mindset for Decentralization
Terumo established a global task force to evaluate and define the core requirements for digital infrastructure in clinical operations. Prior to selection and implementation, they had to make sure that the solution fully represented the needs of all stakeholders in every region.
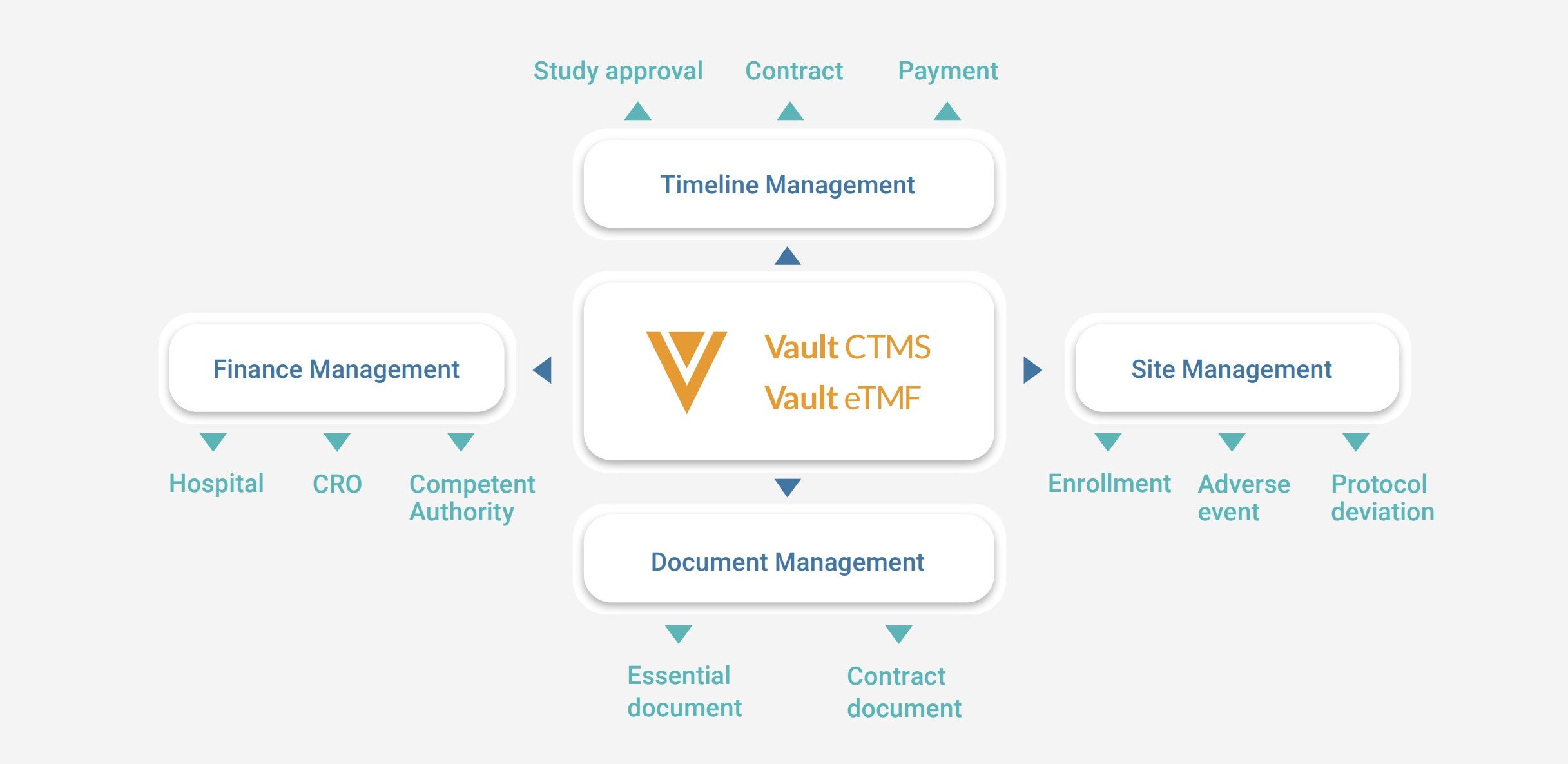
Through the process, the team identified a need for a single CTMS (clinical trial management system) and eTMF (electronic trial master file) to transform their workflows and unify working practices across regions.
“We really needed to come together with a single mindset and collaborate using a single tool,” explains Irene Barriocanal, clinical project manager at Terumo Europe, who coordinated the implementation.
The global task force adopted the brand name ‘Sekai’, from the Japanese word for ‘earth’, to instill the ‘One Team Terumo’ vision, support digitalization, and encourage continuous collaboration among clinical groups. They selected Vault eTMF and Vault CTMS from the Veeva MedTech Vault Clinical Suite as a strong match for their requirements across document management, timeline management, site management, and financial management functions.
Achieving Effective Implementation

Veeva MedTech worked closely with the Terumo taskforce team to develop a global execution strategy. The objectives were to ensure full standardization while minimizing cost, mitigating risks, and adhering to strict timelines. The implementation started in Europe and ultimately cascaded to the regional entities and the Japanese headquarters.
Senshu highlights three success factors from this complex transformation program:
1. Communication and collaboration between internal stakeholders and Veeva MedTech. “There was close communication between all parties about expectations and what improvements were needed to succeed. It was a true partnership,” Barriocanal recalls.2. Strong leadership from Terumo Europe when spearheading the global project. “The most important thing for our success was uncompromised conversations with all stakeholders. Sufficient dialogue meant we could find the best solution for everyone,” adds Senshu.
3. Detailed execution plan created by Veeva MedTech. The team achieved collaborative success while managing the challenges of working virtually throughout the COVID-19 pandemic.
“Our daily life will improve with the implementation of Veeva Vault Clinical Suite. It will allow us to collaborate more closely and standardize our way of working.” – Irene Barriocanal, Clinical Project Manager and Team Leader, Terumo Europ
New Platform for Digital-first Clinical Operations
 With this implementation the Terumo team aims to achieve the following objectives:
With this implementation the Terumo team aims to achieve the following objectives:
1) Enhanced clinical operations capabilities
2) Greater efficiency and productivity
3) Enhanced transparency and improved governance
Barriocanal says, “Our daily life will improve with the implementation of the Vault Clinical Suite. It will allow us to collaborate more closely and standardize our way of working.”
The project also paves the way for further innovation in the future. Senshu notes that the Veeva Vault platform marks the first step in executing the vision for Terumo’s global center of excellence, which will harness best practices and the full potential of integrated technology during decentralized trials.
To learn how Veeva MedTech Clinical Solutions help drive efficiency and speed clinical research visit: www.veeva.com/medtech/