Next Steps for FDA’s Lab Developed Test Proposed Rule
Please note: this post concerns a proposed rule. The requirements of any final rule are subject to change.
On October 3, FDA proposed a new rule to phase out general enforcement discretion for Laboratory Developed Tests (LDTs) and state explicitly that they are in vitro diagnostic (IVD) medical devices, subject to the same regulatory requirements as other IVDs. This change would have a significant impact on LDT manufacturers, requiring proactive steps to ensure compliance with the new requirements.
What is a Lab Developed Test?
LDTs are IVDs for clinical use that are designed for use in a single CLIA-certified laboratory and meet CLIA rules on high complexity testing. Historically, these tests were not subject to the same stringent regulatory requirements as traditional IVD medical devices because they weren’t used in many clinical settings. However, with the advancement of medical technology and manufacturing practices, FDA estimates that 70% of medical decisions are currently based on lab results. Over the years, FDA has become increasingly concerned with the safety and efficacy of these tests and there have been multiple studies and class action lawsuits related to LDTs.
Proposed timelines
The proposed rule includes a multi-year phase-in, beginning with MDR and ending with a new user fee cycle and pre-market submission requirements. During this time, LDT manufacturers would need to comply with specific regulatory actions including medical device reporting, adverse events, corrections, pre-market submissions, and more.
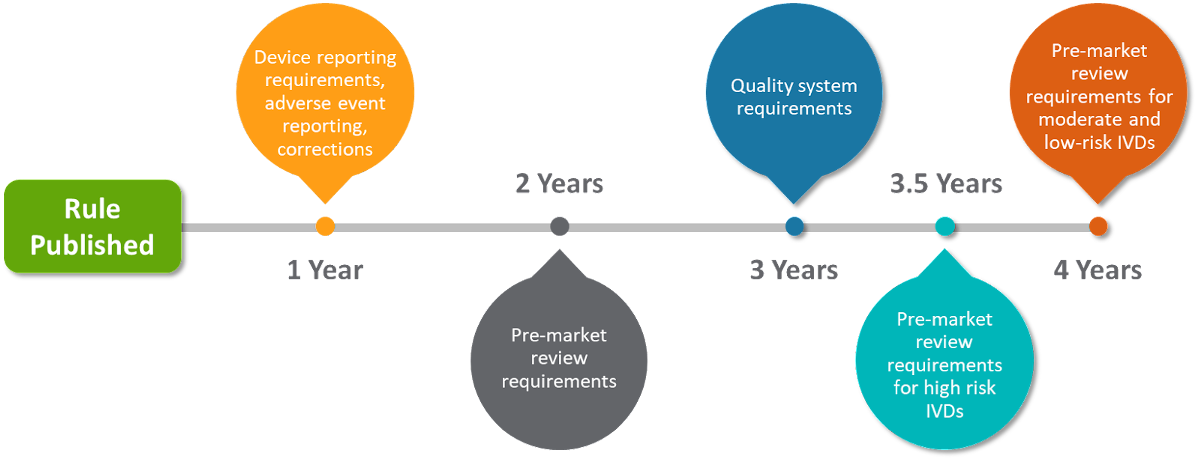
There are already a few exceptions noted by FDA that will allow certain LDTs to continue current compliance measures, including LDTs used in law enforcement, such as specific Human Leukocyte Antigen (HLA) tests, 1976-type LDTs, and tests for public health surveillance.
What to do prior to the rule’s full publication
LDT manufacturers should think critically about the current requirements for IVDs and the proposed timelines for implementation. Will their current processes and staffing levels be able to take on the additional administrative tasks? What is achievable and where may they need to compromise?
If the rule is published, expect that there will be additional changes from the proposed to a final rule. Also, remember that each company will need time to start implementing these changes well before the first phase in order to be compliant.
To learn more about how RA teams can better prepare and adapt to new regulations, including this LDT proposed rule, download the 2023 Veeva MedTech Regulatory Benchmark Report.
—
Additional Resources: