White Paper
Faster Training, Faster Trials: Guide for Site Training
Study training is a high-stakes process. Missing study training documentation can lead to critical inspection findings, resulting in commercialization delays and lost revenue. The potential cost of receiving an FDA Form 483 can easily top $250,000.
Recent FDA warning letters reveal various study training-related challenges at sites, including:
- Role training The investigator and sub-investigators needed more training on study roles and compliance with the delegated tasks.
- SOP training The investigator and other clinical research personnel needed training on applicable SOPs.
- Process training The investigator failed to ensure that study participants met protocol-required inclusion criteria and needed to facilitate training on additional SOPs and internal processes.
- Documentation training Site staff needed training on proper documentation according to Attributable, Legible, Contemporaneous, Original, Accurate, and Complete (ALCOAC) guidelines.
However, sponsors and CROs can implement processes to mitigate these risks and improve the study training they deliver to sites. This guide will assess the regulatory landscape and share tips to streamline and improve site training.
Reduce SIV training time and increase efficiency
In addition to its regulatory implications, study training represents a large hidden cost during clinical trials.
For the average Phase 2 study with 40 sites, site initiation visits (SIVs) cost upward of $140,000 — assuming $3,500 per SIV. It costs an additional $900 on average to train each internal study team member. This means that study training costs more than $200,000 per study, assuming that everyone completes training once and correctly. With retraining, study training costs $350,000 per Phase 2 study.1
In particular, improving SIVs can help sponsors and CROs start on the right foot with sites. Companies can reduce study training costs by administering study training, including protocol training, ahead of the SIV via a purpose-built learning management system (LMS). Picture a scenario in which site staff are gathered in a room, reviewing a PowerPoint presentation with screenshots of each section of the protocol. Then imagine replacing that with an interactive package of online materials, including a video overview of the compound and a quiz on the protocol.
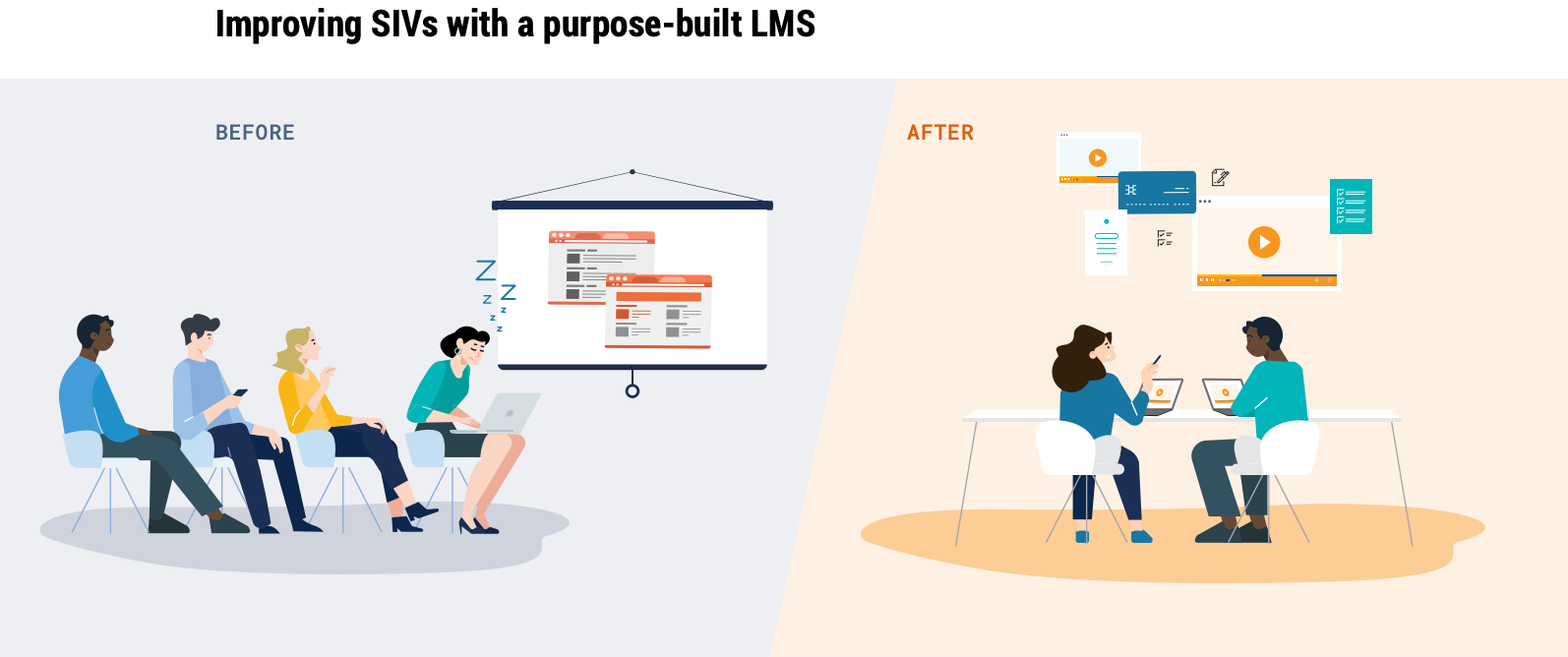

The latter is more effective and efficient, enabling clinical research associates (CRAs) to spend less time on-site administering training. Instead, they can focus on higher-value activities such as:
- Answering PI questions on the protocol or dosing
- Reviewing enrollment criteria
- Building relationships with site staff
Within the purpose-built LMS, CRAs can also easily record proof of completion in a compliant manner. This saves time during the SIV that would otherwise be spent gathering paper signatures. An LMS also makes it easier to account for training of site staff who miss the SIV.
These incremental changes add up. If the sponsor or CRO can shorten the SIV from two days to one with this approach, they can cut the costs of site training in half.
Support sites throughout the study
Sponsors and CROs can similarly improve site study training throughout the course of a trial, better engaging sites in conduct and closeout.
One large biopharma currently uses non-purpose-built systems to deliver training to sponsor and site users. The biopharma’s clinical systems implementation director notes that the systems frustrate users: “The systems weren’t originally built to support protocol training, and users find the systems to be cumbersome and time-consuming.”
They are looking to replace these systems with a purpose-built solution to improve study training, curriculum structure, and user satisfaction.
Increase study training effectiveness with microlearning
Sponsors and CROs can also increase the effectiveness of study training by implementing microlearning methods.
Microlearning improves learner interaction and knowledge retention with shorter, focused, more compelling content delivered through channels like video. It compliments macrolearning, which typically deals with larger, more complex skill areas.
Two key characteristics of microlearning are brevity and specificity. A microlearning experience:
- Has a single, well-defined learning objective
- Focuses on a discrete task, skill, or topic
- Typically takes less than 15 minutes for a learner to complete
For example, this could include a two-minute video overview of a study’s compound. The benefits are clear: Studies show that microlearning results in 85% higher sustained knowledge, especially when used after long-form training.

Here are some practical ways to apply microlearning techniques to more effectively train site staff:

Before and after the SIV, sponsors and CROs can supplement on-site time with microlearning opportunities. This ultimately improves compliance and reduces the risk of protocol deviations, which leads to better patient safety and less retraining.
More effective training will also improve working relationships with sites and enable sponsors to become a site’s sponsor-of-choice.
Learn how you can increase your organization's study training efficiency and effectiveness with Vault Study Training.
1Internal analysis, Veeva Systems, June 2024.