Article
Five Biopharmas and CROs Share How They Benefit From a Modern EDC Across Trial Settings
Traditional electronic data capture (EDC) systems are not flexible enough to address the complex protocols and frequent amendments demanded by today’s clinical trials. These legacy tools hinder study teams by creating hidden data management issues that affect clinical capacity. Data managers using legacy systems have to navigate manual workarounds and custom programming, which cause bottlenecks. This puts pressure on skilled resourcing, delays clinical insights, and increases study costs.
Whether shifting data management activities in-house or asking their contract research organization (CRO) partners to work into different systems, sponsors are finding ways to gain more control over their clinical data. With many users required to undertake migrations or upgrades by their legacy vendors, the time is right to switch to modern and agile EDC systems.
Standardizing across trial settings
While there are many paths to re-gaining data ownership, a standardized data management model is a common success factor.
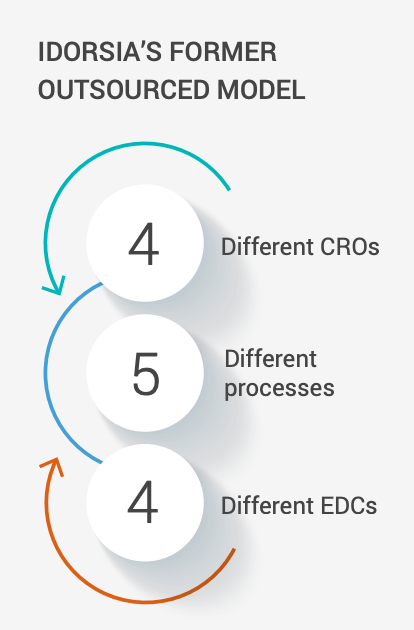
Idorsia Pharmaceuticals, a midsized biopharma company headquartered in Switzerland, used to outsource all its clinical trials. Teams would send study specifications to CRO partners, which were responsible for creating studies in systems, completing user acceptance testing (UAT), and following SOPs, with trial data transferred back to Idorsia. However, the company gradually found that partnering with four CROs using five different EDC systems had fragmented its data management model to the point that oversight was challenging. As Vincent Rolland, director of data management and programming, recalls: “Our standard eCRF (electronic case report form) had to be adjusted to each CRO and EDC system, so we were reinventing the wheel quite often.”
“With this new model, we have all the benefits of standardization and data control, and can train CRO partners on our processes with our SOPs. Vincent Rolland, Director, Data Management and Programming, Idorsia Pharmaceuticals
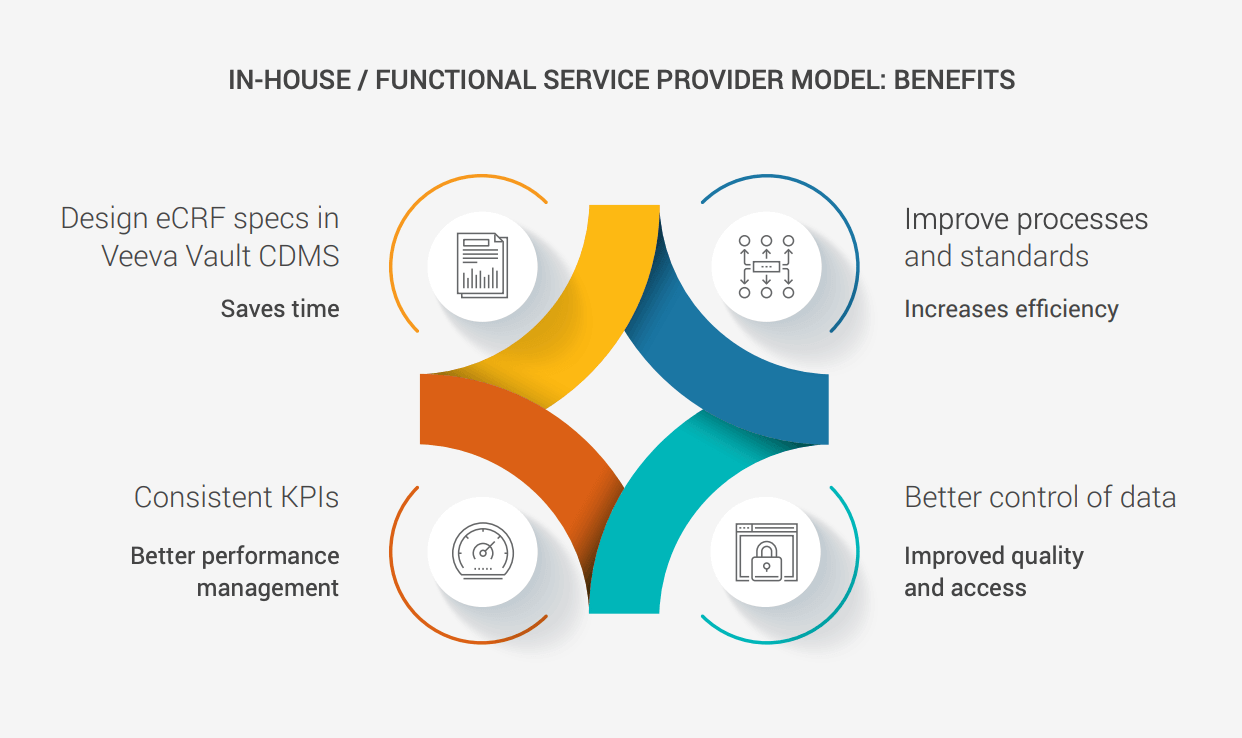
Idorsia chose to bring its EDC in-house, enabling it to design studies directly in Veeva Vault CDMS. The company has accrued efficiencies by using the same tool across all studies and saved time through faster UAT processes, greater use of automation when generating documentation, and fewer edit checks. “With this new model, we have all the benefits of standardization and data control, and can train CRO partners on our processes with our SOPs,” he adds.
Other sponsors have followed a different path to enhance data control through standardization. One midsized biopharma headquartered in Europe outsources the majority of its clinical trials, including many aspects of data management. While implementing Vault EDC, the company built eCRF standards capable of flexing to in-housed, partly, and fully outsourced trial settings. It found that setting up a global library early saves time, especially when working with CROs.
Cumulative gains for sponsors and CROs
Sponsors outsourcing the bulk of their data management activities are the ultimate beneficiaries (or victims) of their partners’ technology choices. When CROs rely on legacy systems that are manually intensive, the results are reflected in slower customer service and higher costs. Conversely, both sides gain when sponsors stipulate which EDC systems they wish their CROs to use.
“We’ve been able to, in some cases, reduce the setup time of an EDC database by up to 50%... And also recognize a 30% cost saving for our sponsors.” Trevor Griffiths, Senior Director, Clinical Data Management, Syneos Health
CRO Syneos Health views technology as an important accelerator of the clinical research it undertakes for biopharma companies. The company has firsthand experience of the difference that a modern EDC system can make to the daily work of its data managers. By moving away from custom functions (and instead configuring dynamic rules and checks within Vault EDC), it has seen a dramatic reduction in study build times. Trevor Griffiths, senior director of clinical data management, comments: “We’ve been able to, in some cases, reduce the setup time of an EDC database by up to 50%... And also recognize a 30% cost saving for our sponsors.”
“That increased efficiency is because the system design allows us to focus on quality.” Jerry Yarem, Vice President, Data Management, Fortrea
Global CRO Fortrea similarly wanted to improve its customer service and competitive position, so decided to unify its clinical infrastructure from EDC and RTSM to clinical trial management software (CTMS) and electronic Trial Master File (eTMF). Embracing a modern EDC enabled the company to minimize programming and time spent on manual activities, as well as reducing its use of unique forms by 20-30%. Jerry Yarem, vice president of data management at Fortrea, observes: “That increased efficiency is because the system design allows us to focus on quality.”
Upgrading to an EDC with advanced and user-friendly build tools also enabled CRO Bioforum to reduce timelines and costs for its customers. “Vault EDC is more agile. It provides an environment where you can deliver a lot faster,” says Tanya du Plessis, chief data strategist and solutions officer. She continues, “Just because you have a complex trial design doesn’t mean you need a complex build. Traditional custom functions cause a lot of delays, not just for builds but for validation and amendments. Working with rules as opposed to custom functions is a game changer. It makes a complex design easy to program.” A modern system architecture helps CROs collaborate with their customers. She continues, “Having sponsors review the system directly instead of via PDFs or printouts is hugely effective. And if sponsors want to make changes, we can show them immediately how that would work. This makes our lives easier and helps us to get it right the first time.”
“Having speed and flexibility in Vault EDC is hugely advantageous for attaining quicker timelines. Amendments are 70% faster with Veeva than other platforms.” Tanya du Plessis, Chief Data Strategist and Solutions Officer, Bioforum
These advantages extend throughout the trial and not just during startup. “Having speed and flexibility in Vault EDC is hugely advantageous for attaining quicker timelines. Amendments are 70% faster with Veeva than other platforms,” she concludes.
End of the road for legacy tools
There are major advantages to shedding legacy technology in favor of a modern and standardized system, and sponsors and CROs are experiencing the benefits across a range of trial settings. As CRFs are built directly from standards and protocols, study builds that used to take months are consistently faster and more streamlined. Amendments without migrations or downtime have addressed a long-standing pain point in modern trials.
“It’s actually quite fun and easy to design your first forms and rules. You save a lot of edit checks because rules are built into the form design. And there’s no migration, hallelujah!” Principal Data Manager, Midsized sponsor
As the principal data manager at the midsized European sponsor observes: “It’s actually quite fun and easy to design your first forms and rules. You save a lot of edit checks because rules are built into the form design. And there’s no migration, hallelujah!”
Learn why biopharma switch to EDC for faster, more efficient studies.