eBook
How Digital Quality Management is Transforming Pharma Manufacturing
Foreword
New-market demands, increasingly complex supply chains, and changing regulations are forcing manufacturers to re-evaluate their disjointed legacy systems and processes. These disconnected systems create business gaps between manufacturing and quality management processes, making it hard to track fast-changing regulations and hit time-to-market goals.
So, how can pharma manufacturing become more agile, data-driven, and future-ready?
Digital transformation is the answer.
Embracing digital quality management, manufacturers can increase agility and collaboration across the supply chain to meet continually changing market demands. Enabling technologies such as cloud computing, artificial intelligence, and the Internet of Things allow manufacturers to scale operations seamlessly for new therapies like precision medicine. And mobile devices running cloud applications can provide operators with real-time access to up-to-date content.
Digital quality management can also streamline discrete quality processes, providing the flexibility needed across the product lifecycle, from clinical to commercial. For example, connecting SOP management with training assignments and delivery enables version-based training automation and faster time to qualification.
The following collection of articles provides quality and compliance teams with a deeper understanding of how digital technologies enable a connected shop floor for better quality products and faster time-to-market.
ARTICLE 1
How digital transformation is meeting the new demands of quality management
The first article in this collection, “Digital transformation: meeting the new demands of quality management in manufacturing,” explains how digital transformation is improving pharmaceutical processes across the industry. Powered by cloud computing, artificial intelligence, and the Internet of Things (IoT), digital quality management enables manufacturers to reliably and economically produce new therapies at scale. Read it here
ARTICLE 2
How a connected shop floor can build operational agility and enable 24/7 manufacturing
The next piece, “Modernizing manufacturing through a connected shop floor,” explores how a digitally connected shop floor can support 24/7 manufacturing and speed production. Electronic devices provide up-to-date content and real-time visibility to operators that drive informed decision-making. The article also highlights specific examples of how mobile devices and intuitive applications can better support modern training methods, improving quality and compliance. Read it here
ARTICLE 3
How Quality 4.0 can transform manufacturing operations
The final article, “Quality 4.0: The Foundation for a New Era of Medicine”, outlines five ways in which Quality 4.0 will improve manufacturing operations, benefitting the life sciences industry. The article reinforces, with specific examples, how Quality 4.0 systems eliminate silos to meet the new demands of quality and manufacturing and support innovation in a new era of medicine. Read it here
Digital transformation is on the rise in the biopharma industry. Companies utilizing advanced digital technologies are affirming leadership positions and becoming more competitive globally.
The ultimate goal is to meet the current and future needs of patients. Modern digital technologies are well on their way to meet those expectations as market and quality demands evolve.
ARTICLE 1
Digital transformation: meeting the new demands of quality management in manufacturing
Advancing digital technology is bringing new opportunities to improve quality and efficiency in the pharmaceutical industry. Many life science organisations are transforming manual and document-centric processes into more data-driven ways of working. They are replacing a formerly siloed system landscape with unified and connected applications.
In pharmaceutical manufacturing this addresses long-standing inefficiencies, placing product supply units in a stronger position to meet future demands. It is important for manufacturing operations to become more agile, while embracing the new paradigm of precision medicine. Enabling the creation of a connected shop floor allows for real-time visibility across data and processes for better tracking and analytics. This is a pre-requisite for adaptive manufacturing and continuous improvement.
Precision medicine transforming processes
Enabling technologies such as cloud computing, artificial intelligence, and the Internet of Things have reached enterprise-scale maturity. However, the real driving force for transformation will be changing market demands. In 2018, the FDA approved 62 new therapeutic drugs, of which 25 were precision medicines.1 This emerging approach to disease treatment targets a patient’s unique molecular and genetic profile and therefore requires changes in the way that products are manufactured. Typically, precision medicines are made for small groups of people with hard-to-treat illnesses. The reality is that they can be complicated to produce and difficult to scale.
Additionally, with many precision therapies, the patient becomes an integral part of a highly specific end-to-end manufacturing process. For example, with chimeric antigen receptor (CAR) T-cell therapy, approved for relapsed and refractory leukaemia and lymphoma, the patient’s cells are collected at the hospital, shipped to a manufacturing centre for engineering to target the person’s specific cancer, and then sent back to the hospital for infusion into that patient.
The opportunity for change
What are the levers to get pharma manufacturing more agile, data-driven, and ready for future demands? As an example, much of the information on the pharmaceutical shop floor is currently buried in paper binders or siloed applications. Without centralised systems for tracking and distributing content and intuitive applications that offer quick access, operators are not able to easily consume the right information they need to perform their jobs. This increases the risk of human errors. Significant overhead is required to ensure that Standard Operating Procedures and Work Instructions remain current and followed by every employee.
In addition to the challenges with document-centric instructions, the systems landscape is mainly disconnected and based on ageing technology. Companies may try to get as much value as they can from their quality management and manufacturing systems by operating them past their shelf life. This practice can actually increase costs because ageing systems tend to be over-engineered, customised, and require frequent maintenance. Older systems are often too rigid to adapt to new processes, manufacturing or training methods, or production requirements, and they cannot efficiently scale down for small batch production required for precision medicines
Matching up to other industries
The life sciences industry has continued to lag behind other industries in the adoption of new technologies. In fact, a recent survey by Deloitte with MIT Sloan Management Review showed that only 20% of biopharma companies are maturing digitally.2 However, meeting the requirements for innovative therapies, such as precision medicines, depends on the industry’s ability to leverage modern solutions. This is key to enabling timely delivery of information to the plant floor and improving agility.
One of Veeva’s customers, a large contract development and manufacturing organization for biologics, is taking a fresh approach to ensure they remain efficient and agile as they scale. Leveraging modern technology and its parent company’s manufacturing expertise and experience, the organization is progressively building larger and more advanced facilities that can run continuously 24 hours a day, seven days a week.
Going mobile for greater plant agility
Mobile devices are ideal for collecting and distributing real-time information to the plant floor and eliminating paper as the main source of information. Using tablets, operators and technicians can deliver updated content and collect data that can be analysed for improved visibility and efficiency. Cloud applications designed specifically for the manufacturing plant floor run on mobile devices and support manufacturing processes, with up-to-date content and seamless integration with quality management systems.
Synchronising content on mobile tablets at each work station has many benefits. First, the content is completely accessible to operators, including for offline viewing. This eliminates having to page through stacks of paper to find the right instructions.
Second, mobile applications provide real-time visibility into quality events, allowing manufacturing and quality teams to address and resolve issues quickly when they first come to light, before they have a bigger impact. For example, with a tablet, workers can detect deviations right on the plant floor and enter them immediately at the point of observation, permitting rapid triaging, impact assessment, and remedial action as quickly as possible.
Human error prevention with improved training methods
A connected shop floor supports training methods that provide the flexibility and versatility needed in modern manufacturing. Information, such as relevant digital procedures and work instructions, can be presented to workers at specific points in the manufacturing process, reducing complexity and, with it, variation.
This targeted learning approach is replacing passive “read and understand” instructions, ensuring measurable training effectiveness. Companies can expect better results from training programs that are shifting from individual, content-driven events to learning that is deeply contextual, social, and embedded into the flow of everyday work.3 This approach ensures that individuals are not just qualified but also prepared to do their jobs.
Training platforms that apply these techniques are catching on in the life sciences industry. By connecting learners with training content at the time of need and according to specific learning styles, companies can change behaviours to decrease quality events. Mapping training content to learner roles based on job functions, then delivering it through a role-based, content-centric experience simplifies training, while making it more cohesive and integrated with quality goals.
The future of quality management
Transforming quality management is key to successfully gaining the agility needed for the production of new therapies. Next-generation solutions that emphasise flexibility and efficiency position manufacturers to reap enormous benefits in simplifying and improving quality management.
To meet current and future needs of patients, life-science companies can enable processes that are flexible and always compliant. Eliminating siloed systems in favour of streamlined solutions allows for greater agility and stronger collaboration, while enhancing compliance and end-to-end control. This will help enable life-science organisations to meet the new demands of quality management in manufacturing and help support innovation in precision medicine.
2 “Survey Finds Biopharma companies lag in digital transformation,” Deloitte Oct. 2018
3 “Corporate Learning Programs Need to Consider Context, Not Just Skills,” Harvard Business Review Nov. 2017
ARTICLE 2
Modernizing manufacturing through a connected shop floor
Eliminating siloed systems in favor of streamlined applications allows for greater agility and stronger collaboration, enabling pharma to meet the new quality management demands
"Companies want to crea te smarter, more agile manufacturing facilities. By digitalizing their content management and delivery, manufacturers can ensure operational alignment and empower teams with access to the information they need." – Jan Paul Zonnenberg, operations management consulting partner for pharmaceutical and life sciences companies, PWC
The shop floor is ripe for digital transformation. Manufacturing operations are still mostly paper-based, with aging systems also in use long past their shelf life. Adapting to new processes, manufacturing or training methods, or production requirements is difficult because processes are manual and systems are rigid or function in silos.
As a result, companies are modernizing manufacturing operations with advanced mobile applications that can bring workstations online and significantly improve agility and efficiency, while maintaining quality and compliance. With a connected shop floor, facilities can support 24/7 manufacturing and manufacturers gain real-time visibility for greater intelligence and smarter decision-making.
“Companies want to create smarter, more agile manufacturing facilities,” said Jan Paul Zonnenberg, operations management consulting partner for pharmaceutical and life sciences companies at PWC, the global auditing and consulting firm. “By digitalizing their content management and delivery, manufacturers can ensure operational alignment and empower teams with access to the information they need.”
Modern cloud solutions are helping companies seamlessly bring together people, processes, and technology – accelerating their digital transformation and transition to a connected shop floor.
Building operational agility
The rise of precision medicines and complexity of supply chains combined with disruptive events, such as natural disasters or political instability, are forcing companies to re-evaluate their business agility.
Precision medicines hold tremendous potential to transform clinical practices. The Food and Drug Administration approved an all-time record 62 new therapeutic drugs last year, of which 25 were personalized medicine therapies. Typically made in small volumes, these therapies can be complicated to produce and difficult to scale. Traditional, large-scale blockbuster drug manufacturing processes are not aligned with the production of highly individualized medicines. The success and scalability of personalized medicine requires new strategies for automation and improved workflows to produce these therapies reliably, safely, and economically.
Going mobile for responsive manufacturing
Today, much of the content on the manufacturing shop floor is only accessible in paper binders or siloed applications. Without digital distribution of procedures and work instructions, it is hard to keep information current when there are frequent updates, or changes when sites or manufacturing lines produce new products. Companies face intense pressure to quickly get finished goods out the door, and any delay impacts revenue.
The life sciences industry continues to lag many other industries in adoption of new technologies. In a recent survey by Deloitte with MIT Sloan Management Review, only 20 percent of biopharma companies are maturing digitally. Leveraging solutions to enable timely delivery of information to the plant floor and tying real-time data with quality management systems can improve agility and help manufacturers meet requirements for innovative therapies such as personalized medicines.
Cloud applications designed specifically for the manufacturing shop floor run on mobile devices and support manufacturing processes with up-to-date content and seamless integration with quality management systems. A connected shop floor can drive greater manufacturing agility, and mobile devices are ideal to deliver and collect real-time information.
Devices such as tablets can deliver updated content to operators as well as provide real-time visibility into quality events so teams can quickly address and resolve issues before they have a bigger impact. For example, deviations detected on the shop floor are immediately entered into a tablet at the point of observation. Instant visibility into deviations and other quality events allows for rapid triaging, impact assessment, and remedial action as quickly as possible.
Companies like Samsung BioLogics, a large contract development and manufacturing organization (CDMO) for biologics, are adopting technology to ensure they remain efficient and agile as they scale. “In the current medicine era, CDMOS must adapt their manufacturing facilities to support multi-drug demands,” said James Choi, chief information officer at Samsung BioLogics. “With modern technology and automated processes, we are reducing the time it takes and number of batch losses suffered when switching between products.”
Enabling 24/7 manufacturing
With paper-based processes, significant overhead is required to ensure the content that workers need to complete tasks is current and easily accessible. Companies that have invested in legacy systems have also realized these solutions cannot reliably support continuous uptime and there are long scheduled downtimes due to system maintenance, upgrades, and re-validation.
"With modern technology and automated processes, we are reducing the time it takes and number of batch losses suffered when switching between products." – James Choi, chief information officer, Samsung BioLogics
It’ s challenging to manage, maintain, and provide continuous access to current information on the manufacturing floor. Delivering content directly to manufacturing stations through a mobile application – that also offers offline access – will ensure operators are always working from the latest procedures and make it easier for sites to run twenty-four by seven. – James Choi, chief information officer, Samsung BioLogics
Shifting to a 24/7 manufacturing facility increases utilization of equipment and sites and enables companies to be more responsive to business demands. Modern cloud solutions are designed to have continuous uptime. Information is always accessible to operators, including for offline viewing. Synchronizing content onto mobile tablets at each work station allows operators to quickly access correct information at the point of need to perform their job. Eliminating paging through stacks of paper to find the right instructions drives greater adoption and increases operator compliance – potentially leading to fewer deviations.
Increasingly, pharma manufacturing sites – especially at contract manufacturing organizations – are transitioning to a continuous operating model. Leveraging modern technology and its parent company’s manufacturing expertise and experience in other industries, Samsung BioLogics is progressively building larger and more advanced facilities that can run continuously 24 hours a day, seven days a week.
“It’s challenging to manage, maintain, and provide continuous access to current information on the manufacturing floor,” said James Choi, chief information officer at Samsung BioLogics. “Delivering content directly to manufacturing stations through a mobile application – that also offers offline access – will ensure operators are always working from the latest procedures and make it easier for sites to run twenty-four by seven.”
Direct visibility
A connected shop floor has the potential to improve productivity and enable better decision-making. Managers track how content is consumed at each facility, station, and device, and update the content on an as-needed basis. This functionality offers a new lens into the effectiveness of the content.
For instance, managers can measure whether document-based instructions are more successful than a short video in engaging workers and improving comprehension. Video is gaining popularity as an effective training tool. Delivering video though mobile devices directly on the shop floor could improve efficiency and compliance. Managers could see how employees are engaging with the content delivered digitally to each station, and, using that data, design instructions and training modules to fit with that particular task or even a particular employee’s learning style.
Centralizing quality event information provides a more complete view and enables greater insights for better decision-making. Data and metrics help identify trends for proactive, and eventually predictive, quality decisions. Teams gain a deeper understanding on how quality events are related, furthering the ability for improving quality and manufacturing operations.
Quality 4.0 and a connected ecosystem
Paper-based process and legacy systems create many business gaps between manufacturing, quality management (QMS), and content management systems, making it challenging to effectively deliver quality products. Innovative life sciences companies are embracing Quality 4.0 technologies to improve operational efficiency and effectiveness and product quality.
Quality 4.0 comes from Industry 4.0 and is usually defined as the adoption of new technology to improve operational efficiency and product quality. Quality 4.0 enables quality systems to integrate seamlessly with complementary systems such as manufacturing execution (MES), enterprise resource planning (ERP), product lifecycle management (PLM), or compliance training systems across the value chain for a more holistic view and seamless execution.
Enabling end-to-end processes helps resolve issues faster. When a MES detects a potential non-conformance, it promptly sends the information to a QMS. The quality team can then quickly evaluate, remediate, or resolve the non-conformance. Connecting a QMS with a MES enables rapid detection, triaging, and remediation of non-conformances.
Connecting operational data allows proactive risk management by addressing quality issues before they arise, as well as provide real-time quality data for analysis to increase productivity and allocate resources based on risk and need. Almost 60 percent of biopharma companies say digital is a top priority and they expect to realize the value of their investments within the next five years. Modern quality management systems also provide transparency for all parties that can drive greater collaboration between employees and suppliers. Information shared with partners helps build alignment and drive progress towards common goals.
With mobile tablets, companies can more easily achieve Quality 4.0 by connecting the shop floor to upstream and downstream systems and all stakeholders.
Modern training techniques
Quality 4.0 can enable companies to better support modern training methods – providing flexibility and versatility needed in today’s manufacturing environment. Information, such as relevant digital procedures and work instructions, presented to workers on a mobile tablet at specific points in the manufacturing process simplifies complexity and reduces variation.
Passive learning in the form of “read and understand” instructions is gradually being replaced with more engaging content that truly helps employees learn how to do their jobs. Companies can expect better results from training programs that are shifting from individual, content-driven events to learning that is deeply contextual, social, and embedded into the flow of everyday work. This ensures individuals are not just qualified but also prepared to do their jobs.
Training platforms that apply these techniques are catching on in the life sciences industry. With Quality 4.0 and mobile tablets, learners can access training content at the time of need and according to specific learning styles – changing behaviors to decrease quality events. Mapping training content to learner roles based on job functions, then delivering it through a role-based, content-centric experience simplifies training, while making it more cohesive and integrated with quality goals.
Connecting the shop floor with mobile devices and cloud-based applications is an example of Quality 4.0 in action. The best Quality 4.0 technologies simplify and speed up manufacturing, while enhancing compliance and quality.
Greater regulatory compliance
By applying technology that we use in everyday life – mobile devices and intuitive applications – manufacturing operations can become more flexible and aligned to the business. Content delivered to the manufacturing floor via mobile applications remains current, and information is gathered in real-time to support quality and compliance.
A pharmaceutical and medical device company that makes specialty products for civilian and military populations is investing in modern technology to transform processes at all eight manufacturing facilities around the globe for greater speed, compliance, and productivity. Open applications are easily integrated to support continuous, controlled processes – eliminating information duplication and strengthening data integrity. “Mobile tablets will eliminate paper and support more contemporaneous data collection,” according to the senior manager, document control and training, at the U.S.-based company.
Digital processes enable sites to fully align with manufacturing needs and be more responsive to new regulatory requirements. Bringing the shop floor closer to decision makers, cloud applications delivered through mobile tablets enables greater agility and better visibility. These modern applications are designed for continuous uptime and allow companies to make configuration changes while drastically reducing the validation burden. Making it easier to stay compliant, companies can keep up with regulatory changes and reduce risk.
The future of manufacturing
Legacy systems and paper-based processes cannot easily adapt to manufacture new products or efficiently scale down to produce smaller volume therapies. Quality 4.0 is becoming a reality in life sciences manufacturing as companies adopt solutions to enable agility while improving operational efficiency and product quality. Adopting Quality 4.0 for a connected shop floor, enables manufacturers to gain real-time visibility across content and quality management processes for better tracking and more meaningful and actionable insights. Life science companies can also easily integrate with internal and external systems that track, engage, and facilitate communication and problem-solving in real-time.
Transforming quality management is key to successfully scaling production of new therapies and is ripe with opportunities. Next-generation solutions that emphasize flexibility and efficiency position manufacturers to reap enormous benefits by simplifying and improving quality management. Samsung BioLogics is already seeing a positive impact with their modern approach, reducing product switching time and enabling greater agility as it scales. As companies shift to multi-product lines, they need to become increasingly nimble.
Flexible processes that maintain compliance and integrate technology solutions can support life sciences companies to meet the needs of patients today and into the future. Eliminating siloed systems in favor of streamlined applications allows for greater agility and stronger collaboration while enhancing compliance and end-to-end control. This will help enable life science organizations to meet the new demands of quality management in manufacturing and support innovation in precision medicine.
ARTICLE 3
Quality 4.0: The Foundation for a New Era of Medicine
As the life sciences industry increases its focus on developing complex therapies and drug shortages grow, Quality 4.0 initiatives are pivotal to transforming manufacturing operations
Pharmaceutical quality and manufacturing teams will face two significant challenges in the coming year.
The first is drug shortages. Recently, the U.S. Food and Drug Administration’s (FDA) Task Force on Drug Shortages shed light on the impact quality has on drug supply. It found that nearly two-thirds of 163 drugs that went into shortage between 2013 and 2017 were a result of supply disruptions associated with manufacturing or quality problems.1
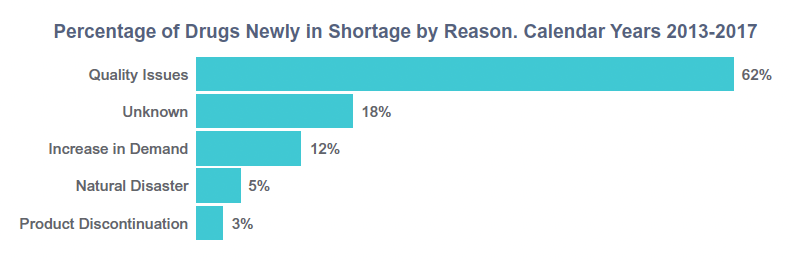
The second issue is the rise of new types of complex therapies, such as precision medicines. Typically made in smaller volumes, these therapies are complicated to develop and manufacture and can create difficulties along the supply chain. Traditional drug manufacturing processes are not suited for these highly individualized medicines.
Quality 4.0 – the digitalization of quality management through technologies that increase operational efficiencies, product quality, and patient safety – provides the foundation for addressing both drug shortages and precision medicine production. With Quality 4.0, companies adopt advanced, digital systems to streamline and automate processes, connect global partners and suppliers, and enable agility that’s so crucial to succeed in a changing regulatory environment.
Some innovative companies such as Samsung BioLogics, a contract development and manufacturing organization (CDMO), are leveraging Quality 4.0 solutions to modernize their quality processes and ensure they remain efficient and maintain quality.
“In the new era of medicine, CDMOs must adapt their manufacturing facilities to support multi-drug demands,” said James Choi, chief information officer at Samsung BioLogics. “With modern technology and automated processes, we are reducing the time it takes and number of batch losses suffered when switching between products.”
5 Ways Quality 4.0 Will Improve Manufacturing
Despite the tremendous potential and the importance of quality on drug supply, only 13.8% of companies have started their Quality 4.0 journey.2 Some organizations are paralyzed not knowing how to get started on the path to Quality 4.0 or the right technologies to adopt. Making this first decision is critical to maintaining the pace of innovation and getting medicines to patients quickly.
Here are five top advantages that Quality 4.0 can bring to the life sciences industry.
1 Increased Scalability to Meet Changing Regulations
The only constant in the regulatory environment is change, yet life sciences companies are limited by rigid legacy systems that cannot adapt easily. With Quality 4.0, however, companies benefit from flexible applications that are easily re-configurable without having to re-validate the entire system. These technologies are also designed for continuous uptime to respond faster to change, reduce risk, and stay compliant.
Cloud-based technologies also bring new regulatory rules and guidance updates from across all 180 countries globally directly into a quality system so manufacturing teams can respond to updates in real time. This seamless connectivity even affords companies the extra time to carefully determine whether a new regulatory rule impacts their specific production line or if they want to set a higher standard than the regulation requires because it impacts quality and patient safety.
2 Greater Visibility of Risk Across Product Lifecycle
Most companies still operate in silos and implement disparate systems across different areas of the business. This limits both visibility and collaboration across the enterprise. Quality 4.0 connects systems and processes to provide greater transparency across the product lifecycle and enable smart decision-making and resource allocation. One area where this has a significant impact is audit management.
With a quality management system that’s connected with other related systems (i.e., the QRM and training applications), manufacturers can define the most pertinent Corrective And Preventive Actions (CAPAs) to holistically address all related audit findings and connect this information to the training curriculum. Further, by connecting audit findings with quality risk management, companies can proactively manage their overall risk profile to meet the evolving expectations of regulatory agencies. This gives companies a clearer understanding of risk upstream during clinical manufacturing and how to make sure those risks don’t become problems downstream during commercial manufacturing.
3 Increased Agility on the Shop Floor
The shop floor is ripe for digital transformation. Manufacturing operations are still mostly paper-based, with aging systems in use long past their shelf life. Adopting new processes for manufacturing or testing methods to meet higher quality requirements is often challenging because of manual processes and rigid systems in silos. Without the implementation of modern technologies that enable digital distribution of procedures and work instructions, it is hard to keep information current when sites or manufacturing lines need to make updates and changes to produce new products.
Quality 4.0 empowers companies to modernize manufacturing operations with advanced mobile applications that can bring workstations online and significantly improve agility and efficiency, while maintaining compliance. With a connected shop floor, facilities can support 24/7 manufacturing and gain real-time visibility for smarter decision-making. Important information is always accessible to operators, even for offline viewing. Synchronizing content onto mobile tablets at each work station also allows operators to quickly access correct information at the point of need to perform their jobs efficiently.
“It’s challenging to manage, maintain, and provide continuous access to current information on the manufacturing floor,” added Choi. “Delivering content directly to manufacturing stations through a mobile application – that also offers offline access – will ensure operators are always working from the latest procedures and make it easier for sites to run 24/7.”
4 Connecting the Manufacturing Ecosystem
Paper-based processes and legacy systems create many business gaps between manufacturing, quality management (QMS), and content management systems, making it challenging to effectively deliver quality products. However, Quality 4.0 brings together complementary systems such as manufacturing execution (MES), enterprise resource planning (ERP), and compliance training systems for a more holistic view and seamless execution.
Connecting end-to-end processes across the manufacturing ecosystem helps resolve issues faster. For instance, when a MES detects a potential non-conformance, it can immediately send the information to a QMS. This enables rapid detection, triaging, and remediation of non-conformances. Additionally, connecting the QMS to the content management and training systems enables timely push of appropriate content into operators’ training curriculum to reduce the incidence of similar non-conformances in the future.
5 Improved Collaboration with Global Partners and Suppliers
Many life sciences companies struggle to work with suppliers and leverage the expertise of external partners worldwide. This is particularly problematic when developing precision therapies and rare disease medications, such as with CAR-T therapies, that often involve an expansive network of partners and suppliers that need to work together and bring these innovative therapies to patients.
Unified systems increase transparency across all parties for greater collaboration between employees, suppliers, and contract partners such as CDMOs. As an example, modern systems allow pharmaceutical manufacturers to automate supplier qualification processes and effectively manage supplier corrective actions (SCARs). Linking SCARs to related deviations from incoming raw materials from the supplier can reduce risk of releasing batches and also save time when evaluating suppliers for future products
“Companies want to create smarter, more agile manufacturing facilities,” said Jan Paul Zonnenberg, operations management consulting partner for pharmaceutical and life sciences companies at PWC, a global consulting firm. “By digitalizing their content management and delivery, manufacturers can ensure operational alignment and empower all partners with what they need.”
The Future of Manufacturing
Manual processes and dated technologies cannot easily adapt to manufacture innovative products or efficiently scale to ensure their reliable delivery to patients. Fortunately, Quality 4.0 is starting to become a reality in life sciences manufacturing as companies adopt solutions to enable agility while improving operational efficiency and product quality. The technologies fundamental to Quality 4.0 initiatives provide real-time visibility across content, data, and quality management processes for better tracking and more meaningful and actionable insights. Life science companies can also easily integrate with internal and external systems to bring all collaborators together.
Next-generation solutions that emphasize flexibility, connection, and visibility position manufacturers for success in the years ahead. Eliminating siloed systems in favor of streamlined Quality 4.0 applications allows for stronger collaboration while enhancing compliance and end-to-end control. This is the key to meet the new demands of quality manufacturing and support innovation in a new area of medicine.
2 LNS Research, “Research Spotlight: Quality 4.0 in Pharmaceuticals,” by Dan Jacobs (November 2018).