White Paper
How to Globalize PSMF Management for Greater Efficiency and Easier Compliance
Easily meet regional regulatory requirements with a modern PSMF solution that harmonizes global content, supports local annexes, and delivers QPPV oversight.
More countries/regions are adopting EU good pharmacovigilance practices (GVP) Module II, however, how they apply the guidance varies greatly by region. This variability makes it increasingly difficult for sponsors to manage the pharmacovigilance system master file (PSMF).
Modern PSMF management solutions have a modular approach that reduces complexity and overhead in maintaining multiple PSMFs while facilitating regulatory learnings between regions. With a single global solution, real-time content visibility, and greater efficiency in PSMF production and maintenance, safety teams can deliver more timely and higher quality PSMFs. They can also leverage this information to improve oversight and to drive improvements in pharmacovigilance (PV) system performance.
Harmonize management and global oversight with one modern PSMF solution
To give regulators a strong first impression of the PV system, keep your PSMF current and available to health authorities in a timely manner. Typically, regulators will want to see the PSMF within a week of their request.
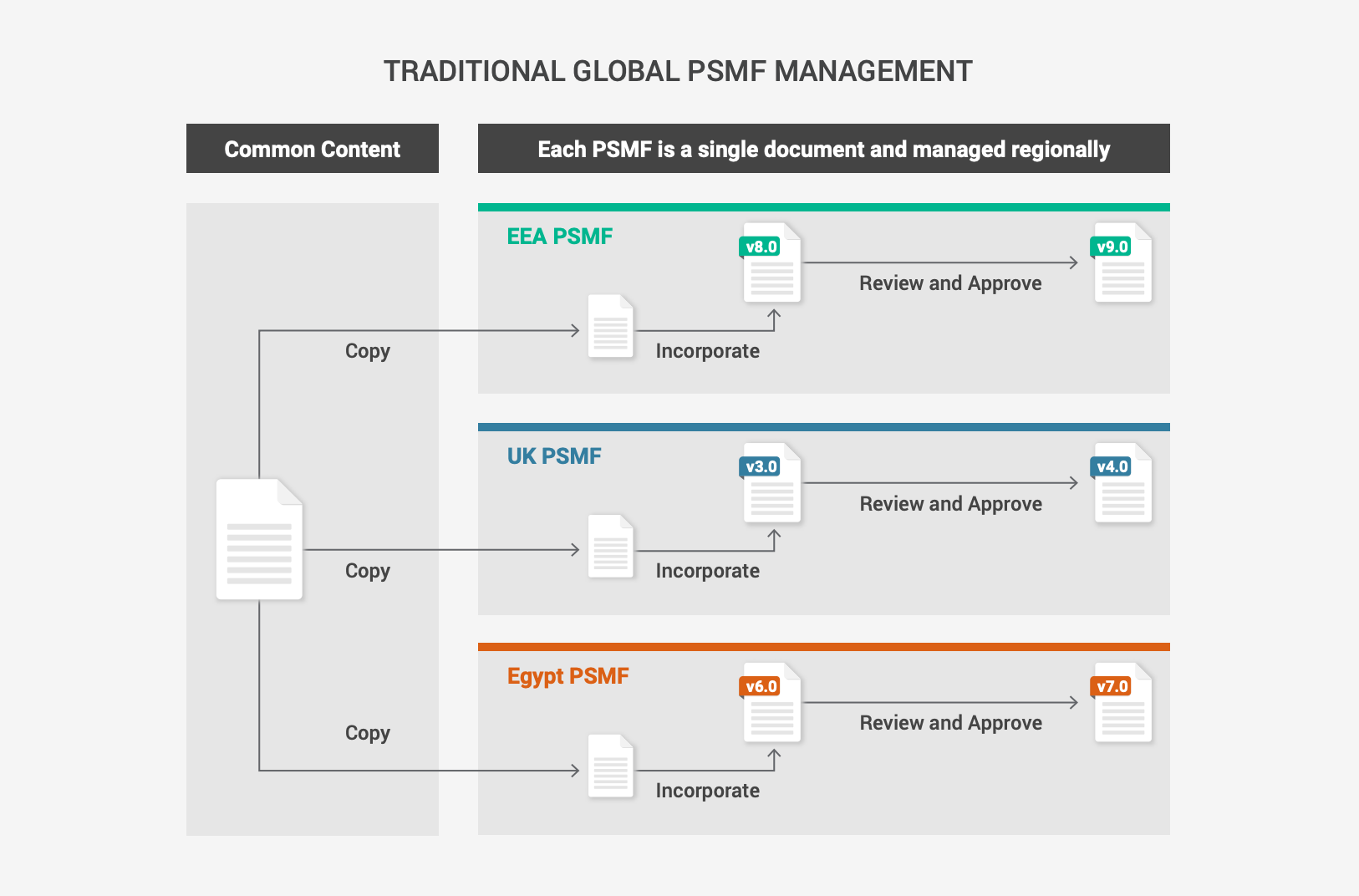
Modular PSMF to support global and regional content
Market authorization holders (MAH) often manage the PSMF at the local level, leading to inconsistent global processes and duplicates of similar documents. Centralizing PSMF management and taking a modular approach harmonizes content across regions. This makes it easier to meet regional requirements while delivering global oversight. Modern PSMF solutions provide a modifiable standard template that is organized into a global main body and annexes, with flexibility for local regulatory and PV experts to supplement the content with local or regional data.
Virtual PSMF binders bring together core content that is applicable to all markets with local or regional documents and data. With one click, the safety team can generate the PSMF. Then, they can collate the core and specific regional content to create the final document for review and approval before submission.
Simplify the IT landscape
Managing content for all PSMFs within one validated GxP solution simplifies the IT landscape. This:
- Eliminates the need to consolidate multiple local in-house or outsourced PSMF systems into one global application
- Reduces cost and maintenance
- Provides greater control and oversight of the content and supporting processes
Empower internal and external content owners to keep documents current
One of the biggest challenges in maintaining a PSMF is keeping content current. The PSMF contains documents owned by different functional areas across the company and partners. Allowing internal or external document owners and subject matter experts to collaborate, update content, and have it dynamically reflected in the PSMF significantly reduces the burden on the qualified person for pharmacovigilance (QPPV).
Modern PSMF solutions have flexible security models that allow companies to provide users access to specific documents or binders and define how they can interact with them – including editing, commenting, reviewing, or approving. Teams can set reminders to ensure content is reviewed periodically according to standard operating procedures (SOPs), and the QPPV team can be notified of changes to support oversight. The QPPV can also track all events in a document’s history in detailed audit trails, including the name of the reviewer or approver and a timestamp that can be shared with an auditor.
Automate logbook entries
Implementing a robust change control will keep your PSMF current and help you stay informed of relevant changes and modifications recorded in the logbook. The QPPV regularly checks the logbook for changes to maintain oversight. With a manual process, it is challenging to keep the logbook up to date, spot which documents were changed, and identify who modified them.
Instead, look for a PSMF solution that automatically tracks changes – including the date, nature of the change, description – and updates the digital logbook. It should also be able to automatically notify the QPPV of PSMF content changes to help them demonstrate oversight during an inspection and generate a more current logbook. Always having an up-to-date logbook eliminates the manual overhead of maintaining another document and reduces compliance risk for the QPPV team.
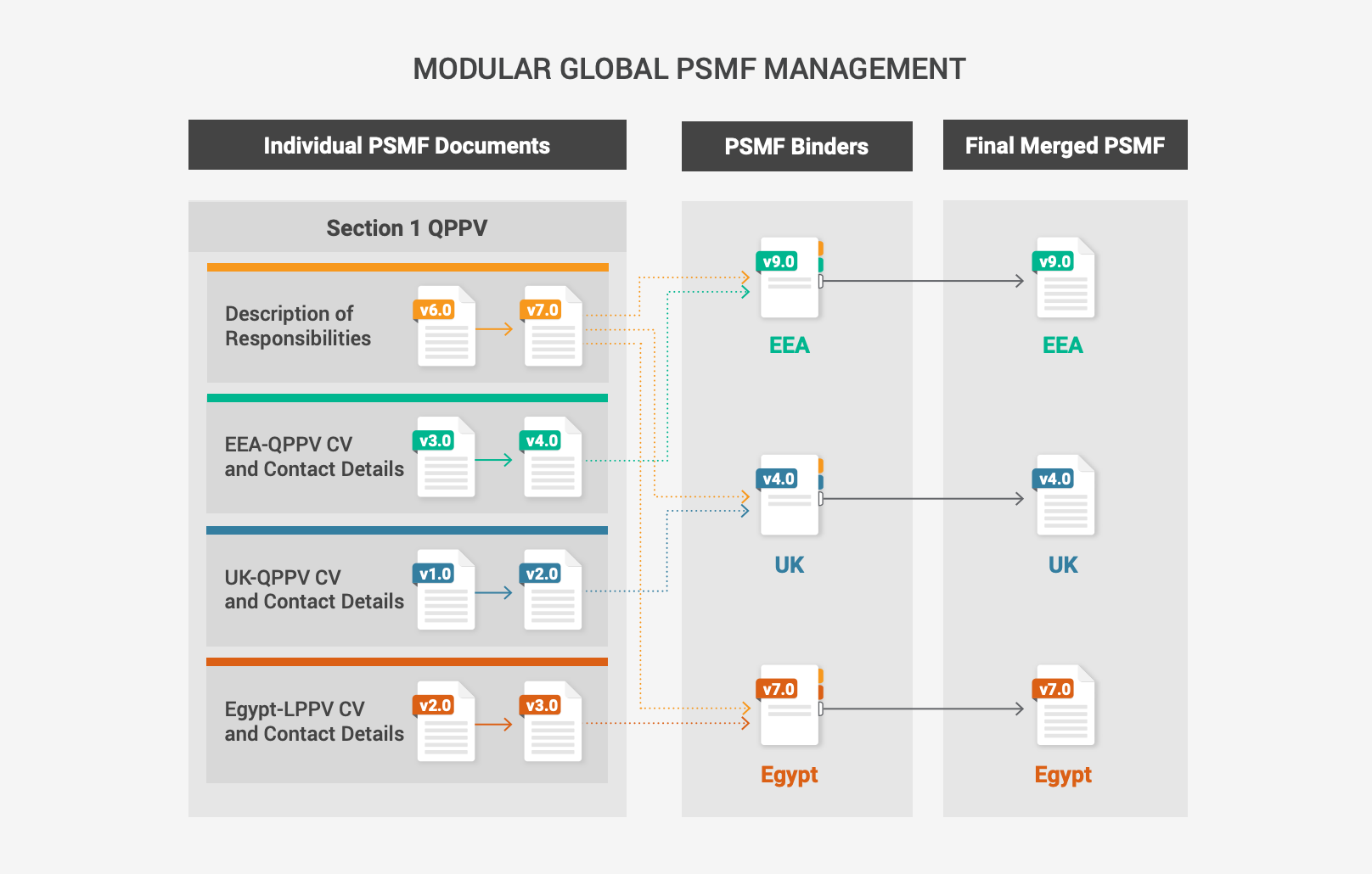
One-click PSMF file generation with digital QPPV approval
Traditionally, producing and maintaining a PSMF is labor-intensive and manual. QPPV teams spend significant time obtaining and compiling documents that must be merged into a single file for submission to a health authority.
A modular PSMF approach allows safety teams to dynamically maintain content and automatically generate the file at any time. This includes the logbook for digital review by the QPPV or the local person for pharmacovigilance (LPPV) before submission. Capturing the approval of the final file with electronic signatures allows companies to demonstrate QPPV oversight in audits or inspections. With a much faster PSMF process, QPPV teams can easily respond to health authorities in a timely manner.
Validated PSMF management solution
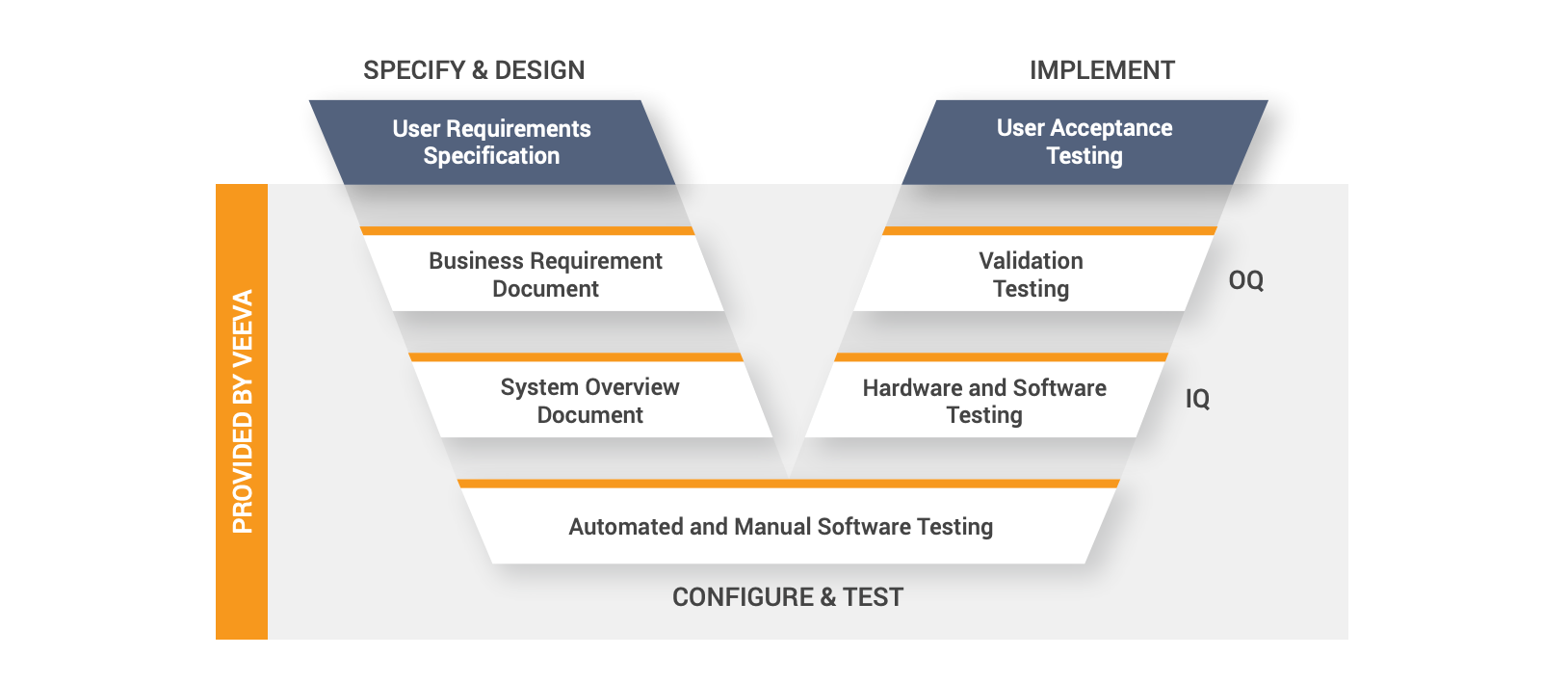
Validating new software applications can be cumbersome and slow. It is even more challenging to maintain a validated environment within a custom solution. This may cause companies to avoid validation altogether by delaying application upgrades.
Opting for a PSMF solution designed for regulated environments will significantly reduce overall validation time and effort. Cloud-based PSMF solution vendors perform and document all elements of infrastructure qualification (IQ) and operational qualification (OQ) for each major release. Sponsors can also leverage user acceptance testing (UAT) scripts and adapt them as needed for performance qualification (PQ). Reducing the validation burden allows even the leanest safety teams to keep up with innovation and new regulatory requirements.
Evolve PSMF into an asset for PV system performance oversight
A harmonized, collaborative approach to PSMF management will lead to:
- Greater efficiency in the production and maintenance of a PSMF
- Higher quality content
- Faster response times to regulatory authorities
- Improved process oversight
One top 10 biopharma cut their cycle time from PSMF authoring to review and approval of the file by 30%. Standardized and modifiable templates with global and local content structures facilitate governance and support oversight with easy navigation and efficient review. Virtual binders enable fast generation of a specific local PSMF. “Previously, it would take me many hours to publish a PSMF,” said a PSMF steward at a top 20 biopharma. “Now, I can produce a merged PDF that contains all of the documents in the correct formatting in 10 minutes.”
With digital end-to-end processes, a modern PSMF solution provides greater control and continuously collects data to enable compliance tracking. For example, automated emails notify content owners of reviews and updates. QPPV teams can leverage reports to track whether content tasks are completed on time. With greater transparency, “We’ll start to see much more engagement from the PSMF contributors when it comes to completing their tasks,” said the top 20 biopharma PSMF steward. Process data can support embedded analytics for the QPPV team, either leveraging existing reports and dashboards or modifying and creating new ones. “Reports give great oversight of the PSMF. You can see what stage in the workflow documents are in, or which contributors still have open tasks and reach out to them,” the PSMF steward said.
Cloud-based solutions are not only transforming PSMF management. They are also changing how content is managed across biopharmas and their external partners. For example, sources for PSMF documents can be owned by departments outside of safety. This causes difficulties identifying content owners and verifying that content is current. With a single cloud platform across functional areas, PV employees can associate documents in any department with a PSMF – eliminating content duplication and ensuring data integrity.
As companies adopt modern PSMF solutions and technologies continue to advance, the PSMF can evolve from a repository of content to an asset for enabling real-time oversight of PV system performance. With greater operational visibility, the PSMF can drive proactive focus into key areas to improve performance and reduce inspection requests and time on site.
Hear how Moderna simplified PSMF management for better compliance and efficiency.